Vanillin
Premium Synthetic Ingredient for Perfumery
Vanillin (CAS 121-33-5) is a high-volume synthetic ingredient with a powerful sweet-gourmand profile, best known for its intensely creamy, powdery vanilla-like aroma. A crystalline phenolic aldehyde, vanillin plays a foundational role in both perfumery and flavor applications. While derived synthetically today—primarily from guaiacol or lignin—it reproduces the characteristic scent of natural vanilla bean extract with greater purity, affordability, and versatility. Used across all fragrance families, it serves as a sweetening agent, fixative, and masking material.
Premium Synthetic Ingredient for Perfumery
Vanillin (CAS 121-33-5) is a high-volume synthetic ingredient with a powerful sweet-gourmand profile, best known for its intensely creamy, powdery vanilla-like aroma. A crystalline phenolic aldehyde, vanillin plays a foundational role in both perfumery and flavor applications. While derived synthetically today—primarily from guaiacol or lignin—it reproduces the characteristic scent of natural vanilla bean extract with greater purity, affordability, and versatility. Used across all fragrance families, it serves as a sweetening agent, fixative, and masking material.
Premium Synthetic Ingredient for Perfumery
Vanillin (CAS 121-33-5) is a high-volume synthetic ingredient with a powerful sweet-gourmand profile, best known for its intensely creamy, powdery vanilla-like aroma. A crystalline phenolic aldehyde, vanillin plays a foundational role in both perfumery and flavor applications. While derived synthetically today—primarily from guaiacol or lignin—it reproduces the characteristic scent of natural vanilla bean extract with greater purity, affordability, and versatility. Used across all fragrance families, it serves as a sweetening agent, fixative, and masking material.
Synthetic Ingredient Overview
🏭 Manufacturer: Multiple global suppliers
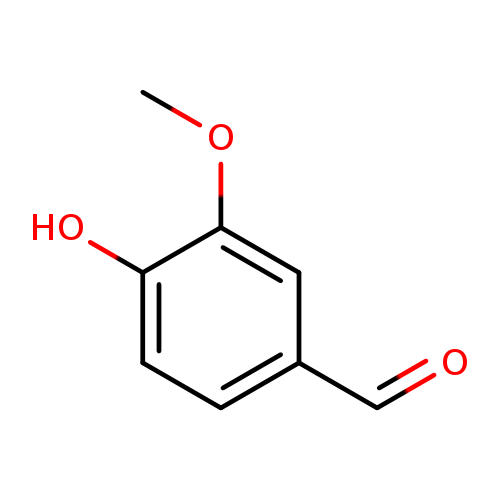
🔎 Chemical Name: 4-hydroxy-3-methoxybenzaldehyde
🧪 Synonyms: Vanilla aldehyde
🧬 Chemical Formula: C₈H₈O₃
📂 CAS N°: 121-33-5
📘 FEMA: 3107
⚖️ MW: 152.15 g/mol
📝 Odor Type: Gourmand
📈 Odor Strength: High; ~400+ hours tenacity
👃🏼 Odor Profile: Sweet, powdery, creamy, phenolic-burnt, almond, spicy
👅 Flavor Profile: Sweet, vanilla, root beer, cream soda, fatty base-dependent
⚗️ Uses: Fixative, sweetening agent, masking material, functional product base
🧴 Appearance: White to off-white crystalline powder or needles
What is Vanillin?
Vanillin is a phenolic aldehyde historically isolated from Vanilla planifolia, now predominantly synthesized for industrial-scale use. It is chemically defined by three reactive groups: hydroxyl (-OH), aldehyde (-CHO), and methoxy (-OCH₃), giving it high reactivity and multifaceted compatibility in perfumery and flavor.
Natural vanilla extract contains hundreds of compounds; vanillin represents the dominant volatile constituent. Due to limited global vanilla bean supply and high extraction costs, vanillin has become the primary alternative for imparting "vanilla" character.
The material appears as white needle-like crystals, and sublimates when heated. It is stable in a variety of formulation systems and presents one of the most tenacious sweet notes available to perfumers.
Olfactory Profile & Perfumery Applications
Vanillin provides an instantly recognizable aroma: sweet, warm, creamy, and tenacious. It has a "burned" or phenolic character when undiluted, but even trace concentrations deliver a lasting impression.
Key fragrance uses:
Oriental & Gourmand bases: Adds edible warmth, enhances lactonic and amber notes
Florals (esp. Muguet, Heliotrope, Gardenia): Supports creamy-powdery diffusion
Woods & Resins: Balances dry, sharp profiles with a round sweet contrast
Soaps & Detergents: Common at reduced levels; now improved to avoid discoloration
Industrial masking: Used in synthetic rubbers, fiberglass, plastics
Vanillin blends well with coumarin, heliotropine, tonka absolute, cinnamates, salicylates, and many fruit esters. However, it can cause discoloration, particularly in white or pale bases when combined with anthranilates, indoles, or trace metals like iron.
Usage note: Despite widespread use, concentrations above 5–8% in finished products can cause functional issues (e.g., color shifts or soap incompatibility).
Industrial & Technical Uses
Flavor industry:
Vanillin dominates the sweet flavor sector. It's a staple in vanilla, butter, chocolate, and root beer profiles. Used at levels from 50 to 1000 ppm in food; up to 20,000 ppm in icings and toppings. Often evaluated in milk with 12% sugar for clarity in tasting.
Pharmaceutical and Personal Care:
Used in cough syrups, toothpaste, chewing gum, and antiseptic mouthwashes. It also serves in drug flavor masking and as a mild antimicrobial.
Functional Products:
In soaps, detergents, air care, candles—improves olfactory profile of functional products while offering low cost per impact unit.
Solubility:
1% in water, 5% in glycerin, fully soluble in alcohol, oils, and diluted glycols. Less soluble in highly concentrated ethanol.
Regulatory & Safety Overview
IFRA Restrictions: No current restrictions
EU Cosmetic Regulation (Annex III): Requires declaration if concentration >0.001% in leave-on, >0.01% in rinse-off
FEMA GRAS: FEMA No. 3107
ECHA Status: Not classified as hazardous at normal concentrations
Photostability: Phenolic; prone to discoloration in light and air exposure
Toxicology: Mild sensitization potential, but low overall toxicity. Not recommended in iron-containing formulations.
Always consult SDS and IFRA certificate before incorporation into final product.
Additional Information
Synthetic Pathways:
From Lignin (most used):
Enzymatic degradation of coniferyl alcohol → oxidation → Vanillin. Yields high-purity, white crystalline vanillin.From Guaiacol (fully synthetic):
Via chloroform or chloral reactions followed by hydrolysis. Economically scalable and widely used.From Eugenol (clove oil):
Through iso-eugenol and acetyl intermediates. Considered a more “natural” synthetic version.From Safrole (Asia-specific):
Routes via iso-safrole and chavibetol. Not common in Western production due to regulation.
Terminological Note:
Despite its name, “Vanillin” is what most people associate with “vanilla” due to common exposure to synthetic flavorings in food. When diluted, consumers often report "chocolate" or “ice cream” associations before identifying vanilla itself.
Conclusion
Vanillin remains one of the most important and tenacious synthetic materials in the fragrance and flavor industries. Despite regulatory challenges, discoloration issues, and its phenolic behavior, its sweet, edible warmth is unmatched in versatility. Whether in florals, ambers, or industrial scent masking, vanillin plays a foundational role in shaping modern olfactory design.
Sources
Perfume and Flavor Chemicals, S. Arctander
Aromatizzazione, Fenaroli
NCBI PubChem – CID 1183, Vanillin
Fulvio Ciccolo Archives
FEMA GRAS Database
The Chemistry of Fragrance, D.H. Pybus & C. Sell