Maltol ( Veltol )
Premium Synthetic Ingredient for Perfumery
Maltol (CAS 118-71-8), also known as Veltol®, is a synthetic compound belonging to the pyrone family, prized for its intensely sweet, warm, and caramellic scent. Found in crystalline form, it delivers a signature pineapple-strawberry aroma with a toasted-sugar nuance, ideal for gourmand profiles. Though typically used at low levels, Maltol offers exceptional power in sweetening rose compositions, enhancing pine needle notes, and providing volume and diffusionto fruit-floral accords.
Its dual relevance in both flavor chemistry and fine fragrance marks it as a crossover ingredient of enduring value.
Premium Synthetic Ingredient for Perfumery
Maltol (CAS 118-71-8), also known as Veltol®, is a synthetic compound belonging to the pyrone family, prized for its intensely sweet, warm, and caramellic scent. Found in crystalline form, it delivers a signature pineapple-strawberry aroma with a toasted-sugar nuance, ideal for gourmand profiles. Though typically used at low levels, Maltol offers exceptional power in sweetening rose compositions, enhancing pine needle notes, and providing volume and diffusionto fruit-floral accords.
Its dual relevance in both flavor chemistry and fine fragrance marks it as a crossover ingredient of enduring value.
Premium Synthetic Ingredient for Perfumery
Maltol (CAS 118-71-8), also known as Veltol®, is a synthetic compound belonging to the pyrone family, prized for its intensely sweet, warm, and caramellic scent. Found in crystalline form, it delivers a signature pineapple-strawberry aroma with a toasted-sugar nuance, ideal for gourmand profiles. Though typically used at low levels, Maltol offers exceptional power in sweetening rose compositions, enhancing pine needle notes, and providing volume and diffusionto fruit-floral accords.
Its dual relevance in both flavor chemistry and fine fragrance marks it as a crossover ingredient of enduring value.
Synthetic Ingredient Overview
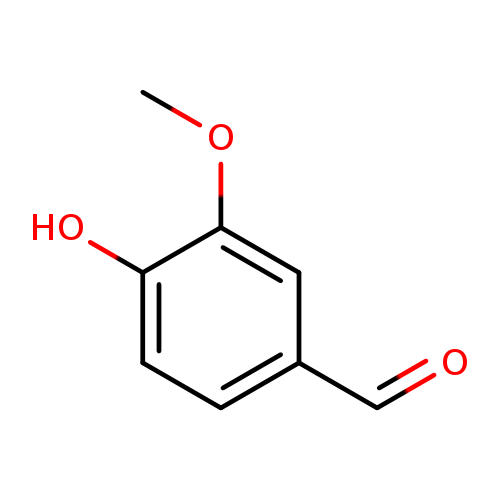
🔎 Chemical Name: 3-Hydroxy-2-methyl-4-pyrone
🧪 Synonyms: Maltol, Veltol®, 2-Methyl-3-hydroxy-4H-pyran-4-one
🧬 Chemical Formula: C₆H₆O₃
📂 CAS N°: 118-71-8
📘 FEMA: 2656
⚖️ MW: 126.11 g/mol
📝 Odor Type: Gourmand, Fruity
📈 Odor Strength: Medium
👃🏼 Odor Profile: Strawberry, pineapple, caramel, cotton candy
⚗️ Uses: Modifier, sweetening agent, top booster for rose and pine
🧴 Appearance: White crystalline solid
What is Maltol?
Maltol is a pyrone-based heterocyclic compound developed to reproduce the naturally sweet and caramelized aroma found in heat-processed foods. It imparts a diffusive, yet gentle aroma, evoking sugarcane, cotton candy, toasted grains, and overripe fruit. Its scent falls between strawberry-laced caramel and the burnt crust of pineapple jam, making it a go-to material for perfumers seeking to construct gourmand or “edible” olfactory illusions.
The molecule binds well to receptors involved in both olfaction and taste perception, producing a synesthetic effectwhere sweetness is not only smelled but felt. As such, Maltol functions as a multi-sensory booster, harmonizing aldehydic harshness or floral thinness with warmth and body.
Historical Development and Natural Origin
Historically, Maltol was first identified in larch tree bark (hence its name, maltol = malt + larch origin), later found in roasted malt, pine needles, and cooked sugar-based foods. Early 20th-century chemists noticed its distinctive caramelic profile and began isolating it during studies of Maillard reaction by-products.
Its recognition as a flavor compound advanced rapidly due to its low toxicity, extremely low threshold, and high sensory impact. Maltol soon became a benchmark for the development of ethyl maltol, a more potent derivative introduced in the 1970s.
According to LeBlanc & Akers, the commercial evolution of Maltol represents a landmark in natural mimicry via synthetic design: a compound structurally similar to natural materials but made reproducible, stable, and cost-effective for modern industries. Its popularity helped bridge early divisions between the fragrance and flavor worlds, positioning Maltol as an early example of a cross-industry molecule.
Synthesis and Industrial Production
Maltol is now manufactured synthetically through several industrial pathways, primarily derived from furfural derivatives, furfuryl alcohol, or pyruvic acid intermediates. Common methods include:
Cyclization of β-dicarbonyl compounds with aldehydes
Oxidative degradation of methyl-substituted furans
Decarboxylative condensation using metal catalysis
These processes allow for scalable yields and high-purity crystalline output. The final material is thermally and chemically stable, allowing easy formulation into:
Alcohol-based fine fragrances
Food and beverage flavorings
Cosmetic emulsions
According to Chemistry and Technology of Flavors and Fragrances, purification to >99% is essential to eliminate trace metallic or sulfurous notes that could distort the otherwise smooth sweetness of the molecule.
Olfactory Profile & Perfumery Applications
Maltol’s unique structure allows it to modulate sweetness perception across olfactory registers:
Top notes: Enhances strawberry, pineapple, banana
Heart: Adds depth to rose, muguet, and heliotrope
Base: Sweet caramel, sugar crust, subtle vanilla warmth
It is used to:
Round out aldehydes and synthetic florals
Sweeten pine-needle accords without terpenic sharpness
Build gourmand bases in lipstick-type perfumes, candy-fruit florals, and lactonic compositions
Amplify diffusion of otherwise quiet blends at <0.05% usage level
Classic pairings include methyl anthranilate, ethyl vanillin, benzyl acetate, and ionone alpha, yielding an expanded fruity-floral character with “sticky” persistence.
Flavor and Sweetening Role
Maltol is GRAS and registered under FEMA 2656. It is primarily a flavor enhancer, not a sweetener per se. It potentiates sweetness by activating the same G-protein coupled receptors stimulated by sugars.
Used in:
Confectionery (chewing gum, toffee, chocolate syrup)
Fruit drinks (especially pineapple, mango, strawberry, guava)
Cereal bars, baked goods, dairy desserts
Cough syrups and children's medicine for masking bitterness
According to Fenaroli’s Handbook, it is frequently used in flavor systems designed for flavor masking, sweetness rounding, and thermal flavor enhancement (e.g., “oven-baked” taste notes).
Typical dosage range:
50–800 ppm depending on matrix
Combined with ethyl maltol for synergistic effects
Crystallinity and Purity Considerations
Maltol is typically supplied in its anhydrous crystalline form, essential for:
Consistent olfactory performance
Accurate dosage metering
Long-term storage and solubility control
The crystal lattice also minimizes spontaneous degradation or oxidation, providing a shelf-stable material for both cold blending and heat-processed formulations.
Formulators prefer pure crystal-grade maltol over diluted liquid variants for:
Better diffusion in alcohol-based perfumes
Controlled release in solid or cream-based carriers
Avoidance of caramelization by-products under high-temperature applications
Storage note: Hygroscopic in open air; store in sealed containers in low-humidity environments to maintain structural integrity.
Regulatory & Safety Overview
FEMA GRAS Number: 2656
IFRA Classification: Permitted at low concentrations; no current restrictions
REACH/ECHA: Fully registered; no hazardous classification
Toxicological profile:
Oral LD50 > 3600 mg/kg (rat)
Non-irritant to skin at standard concentrations
Avoid inhalation of powdered form
Environmental note: Readily biodegradable; no known aquatic toxicity
Sources
Mosciano, Gerard – P&F 17(4), 33 (1992)
Arctander, S. – Perfume and Flavor Chemicals Vol. 1
LeBlanc & Akers – “From the Larch Tree to Food Additive”
Chemistry and Technology of Flavors and Fragrances
Fenaroli’s Handbook of Flavor Ingredients – Burdock, 6th ed.
Sigma-Aldrich Datasheets
PubChem Compound Summary – CID 8369