Amarocit / Methyl Pamplemousse
Premium Synthetic Ingredient for Perfumery
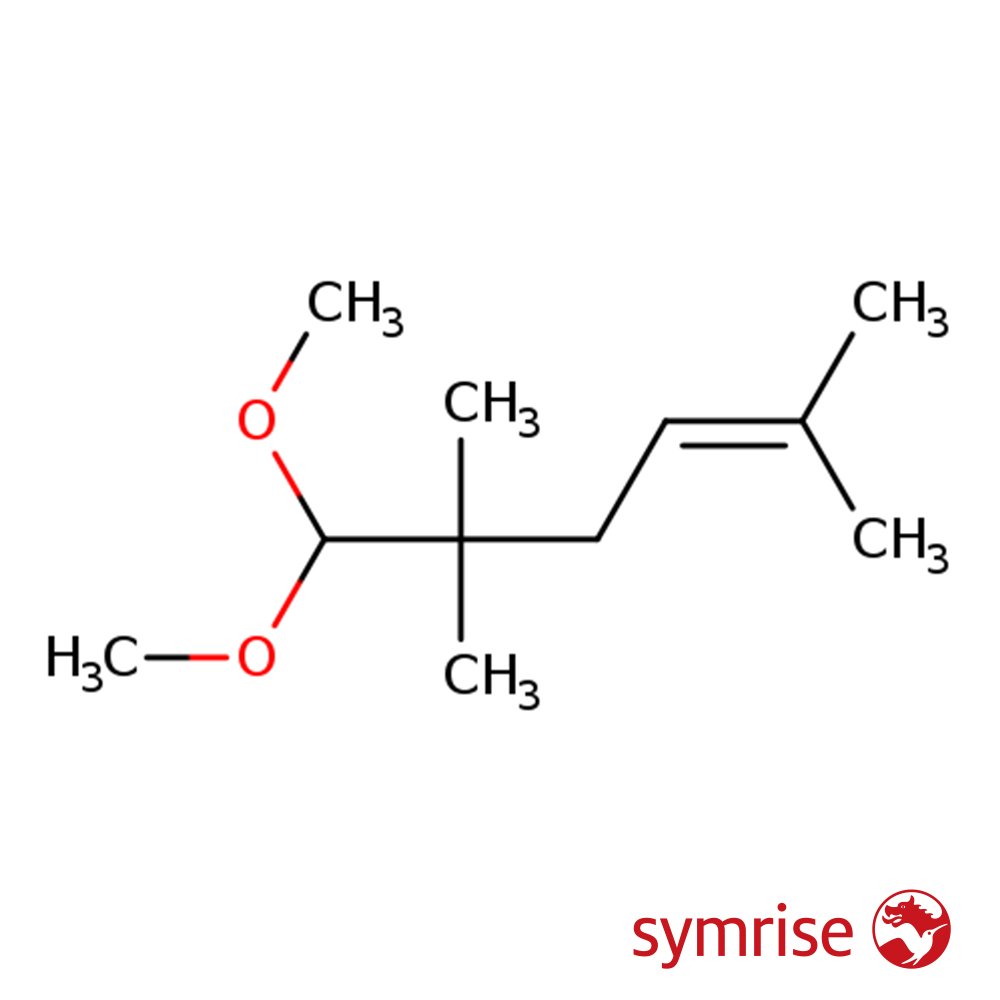
Amarocit (CAS 67674-46-8), also known as Methyl Pamplemousse or Grapefruit Acetal, is a synthetic fragrance ingredient valued for its fresh, bitter-citrus signature with a rosy-fruity nuance. Developed by Symrise and also commercialized under the name Methyl Pamplemousse by Givaudan, it is structurally an acetal that mimics the zest of grapefruit with added complexity and lift.
Amarocit is widely used to modernize citrus top notes, sharpen cologne structures, and reinforce the bitter-green aspects of vetiver compositions.
Premium Synthetic Ingredient for Perfumery
Amarocit (CAS 67674-46-8), also known as Methyl Pamplemousse or Grapefruit Acetal, is a synthetic fragrance ingredient valued for its fresh, bitter-citrus signature with a rosy-fruity nuance. Developed by Symrise and also commercialized under the name Methyl Pamplemousse by Givaudan, it is structurally an acetal that mimics the zest of grapefruit with added complexity and lift.
Amarocit is widely used to modernize citrus top notes, sharpen cologne structures, and reinforce the bitter-green aspects of vetiver compositions.
Premium Synthetic Ingredient for Perfumery
Amarocit (CAS 67674-46-8), also known as Methyl Pamplemousse or Grapefruit Acetal, is a synthetic fragrance ingredient valued for its fresh, bitter-citrus signature with a rosy-fruity nuance. Developed by Symrise and also commercialized under the name Methyl Pamplemousse by Givaudan, it is structurally an acetal that mimics the zest of grapefruit with added complexity and lift.
Amarocit is widely used to modernize citrus top notes, sharpen cologne structures, and reinforce the bitter-green aspects of vetiver compositions.
Technical Ingredient Overview
🔎 Chemical Name — 2,6-Dimethyl-2-hepten-4-one dimethyl acetal
🧪 Synonyms — Amarocit, Methyl Pamplemousse, Grapefruit Acetal
🧬 Chemical Formula — C11H22O2
📂 CAS — 67674-46-8
📘 FEMA — 3743
⚖️ MW — 186.29 g/mol
📝 Odor Type — Citrus, fruity
📈 Odor Strength — Medium
👃🏼 Odor Profile — Bitter grapefruit, sulfurous, green, slightly woody
⚗️ Uses — Citrus reconstitution, top notes, bitter accents, specialty accords
🧴 Appearance — Colorless to pale yellow liquid
What is Amarocit?
Amarocit, known commercially as Methyl Pamplemousse or Grapefruit Acetal, is a synthetic fragrance material belonging to the acetal family. It is prized in perfumery for its unique contribution to citrus compositions, especially those replicating grapefruit peel. This molecule offers a characteristically bitter, sulfurous, and zesty grapefruit nuance that brings realism and complexity to citrus accords. Its structure as a dimethyl acetal of 2,6-dimethyl-2-hepten-4-one contributes to its relative chemical stability compared to natural grapefruit oil components.
Historical Background
The molecule was developed to overcome the limitations of natural grapefruit oil, especially in terms of phototoxicity and oxidative instability. While the precise origin of Amarocit’s first synthesis is not widely documented in open literature, its functional use in perfumery gained traction during the 1970s–1980s alongside other synthetic citrus mimetics. Its commercial adoption reflects the industry's broader turn toward synthetics that could faithfully reproduce citrus freshness without the degradation issues of natural citrals and nootkatone derivatives (Sell, 2006).
Olfactory Profile
Scent Family: Citrus – Bitter
Amarocit imparts a realistic bitter grapefruit note, often described as more rind-like and pithy than sweet. It differs significantly from sweeter limonene-based citrus notes, offering instead a fresh, biting sulfuric tone akin to the oily burst from freshly peeled pink grapefruit skin.
Main Descriptors: Bitter grapefruit, sulfurous, green, rind-like, sharp
Tenacity: Short to medium (1–3 hours)
Volatility: High
Fixative Role: Minimal, mainly a top-note enhancer
Applications in Fine Fragrance
Amarocit is primarily used in:
Citrus colognes
Summer eau fraîche and eaux de toilette
Fruity modern chypres
Tonic and aromatic fougères
It works exceptionally well when blended with:
Nootkatone, Florhydral, Rhubafuran, Allyl Amyl Glycolate
Citrus aldehydes (e.g., Decanal, Aldehyde C10)
Green notes (e.g., cis-3-Hexenol, Stemone)
Notable fragrances including similar grapefruit reconstructions often employ Amarocit or analogous molecules for their bitter edge, though specific formula citations remain confidential in commercial perfumery.
Performance in Formula
Blending Behavior: Amarocit integrates smoothly with other top-note materials and enhances the diffusion of citrus blends.
Diffusion: Excellent, contributing an immediate sparkle.
Fixative Strength: Low, but can benefit from anchoring with white musks or woody notes.
Compatibility: Broadly compatible with both naturals and synthetics in the citrus-green-aromatic spectrum.
Industrial & Technical Uses
Beyond fine fragrance, Amarocit finds limited application in:
Flavor formulations (FEMA 3743) — used sparingly to add bitter citrus zest in beverages and candies.
Functional perfumery — soaps, shampoos, and air care products where a fresh citrus opening is desired without phototoxicity risks.
Its acetal structure lends it greater oxidative stability than terpene-rich natural citrus oils, making it favorable in high-exposure products.
Regulatory & Safety Overview
IFRA (51st Amendment): Not restricted.
GHS Classification: Generally not classified as hazardous; however, always consult specific SDS for manufacturer-specific handling data.
EU Cosmetics Regulation: Allowed; not listed among allergens requiring declaration.
REACH: Registered under REACH for use in fragrance compositions.
FEMA: Approved under FEMA No. 3743 for use in flavor applications with prescribed limits.
No phototoxic or sensitization concerns have been commonly reported at standard usage levels. As always, formula-specific toxicological evaluations are recommended.
References
Sell, C. S. (2006). The Chemistry of Fragrances: From Perfumer to Consumer (2nd ed.). Royal Society of Chemistry.
Arctander, S. (1969). Perfume and Flavor Chemicals (Aroma Chemicals). Montclair: Self-published.
PubChem. (2024). 2,6-Dimethyl-2-hepten-4-one dimethyl acetal. Retrieved from https://pubchem.ncbi.nlm.nih.gov
FEMA. (2024). Flavor Ingredient Library. Retrieved from https://www.femaflavor.org/flavor-library