Schiff 001 - Aurantiol ®
Premium Synthetic Ingredient for Perfumery
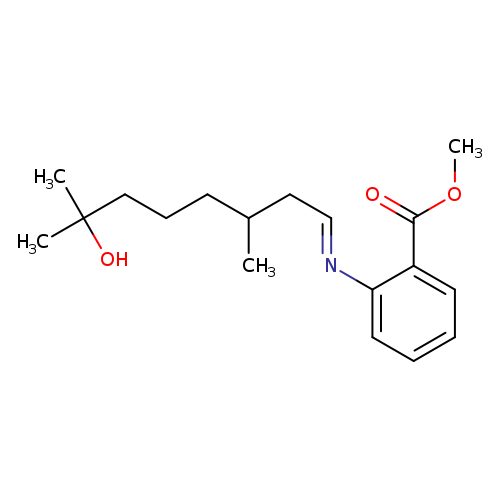
Schiff 001, commonly known as Aurantiol, is a classic Schiff base formed by the condensation of hydroxycitronellalwith methyl anthranilate. This high-impact ingredient exhibits a powerful floral-orange blossom aroma and is widely used in citrus cologne types as both a fixative and top-note enhancer. It also finds use in amber and chypre compositions, where it lends a soft oriental nuance. Schiff 001 is known for its excellent synergy with macrocyclic musks, contributing to round, long-lasting scent profiles.
Premium Synthetic Ingredient for Perfumery
Schiff 001, commonly known as Aurantiol, is a classic Schiff base formed by the condensation of hydroxycitronellalwith methyl anthranilate. This high-impact ingredient exhibits a powerful floral-orange blossom aroma and is widely used in citrus cologne types as both a fixative and top-note enhancer. It also finds use in amber and chypre compositions, where it lends a soft oriental nuance. Schiff 001 is known for its excellent synergy with macrocyclic musks, contributing to round, long-lasting scent profiles.
Premium Synthetic Ingredient for Perfumery
Schiff 001, commonly known as Aurantiol, is a classic Schiff base formed by the condensation of hydroxycitronellalwith methyl anthranilate. This high-impact ingredient exhibits a powerful floral-orange blossom aroma and is widely used in citrus cologne types as both a fixative and top-note enhancer. It also finds use in amber and chypre compositions, where it lends a soft oriental nuance. Schiff 001 is known for its excellent synergy with macrocyclic musks, contributing to round, long-lasting scent profiles.
Synthetic Ingredient Overview
🔎 Chemical Name: Aurantiol (Schiff base of hydroxycitronellal + methyl anthranilate)
🧪 Synonyms: Schiff 001, Aurantiol, Orange Blossom Schiff Base
🧬 Chemical Formula: C₁₈H₂₇NO₃
📂 CAS N°: 89-43-0
📘 FEMA: Not applicable
⚖️ MW: 305.4 g/mol
📝 Odor Type: Floral (Orange Blossom, Sweet)
📈 Odor Strength: High
👃🏼 Odor Profile: Floral-orange blossom, slightly sweet, powdery, faintly balsamic
⚗️ Uses: Top note enhancer, fixative, orientalizer, floral booster
🧴 Appearance: Pale yellow to amber viscous liquid
What is Schiff 001 (Aurantiol)?
Aurantiol (Schiff 001) is a classic example of a Schiff base, a chemical structure formed by the condensation of an aldehyde or ketone with a primary amine. In this case, hydroxycitronellal (a floral-green aldehyde) reacts with methyl anthranilate (a grape-like aromatic amine) to form a less volatile, more tenacious imine derivative.
Schiff bases were first described by Hugo Schiff in 1866, and their relevance in perfumery lies in their ability to form stable, high-boiling aroma chemicals with layered olfactory behavior. Aurantiol exemplifies this: its aroma is long-lasting, smooth, and reminiscent of orange blossom absolute, with soft fruity-floral nuances and balsamic undertones.
Because Schiff bases hydrolyze slowly in formulation, they can release the parent materials over time, contributing to extended fragrance diffusion.
Olfactory Profile and Perfumery Applications
Schiff 001 is known for its intense floral-orange blossom character. It is highly diffusive and finds widespread use in:
Citrus colognes: As a top-note fixative and sweet floral body
Amber and chypre compositions: Contributes an oriental-floral warmth
Soapy or powdery florals: Complements jasmine, neroli, heliotrope, and rose
Musk-forward fragrances: Provides volume and linkage between floral and musky elements
It blends particularly well with macrocyclic musks, which round out its sweetness and enhance longevity without compromising freshness.
Additional Section: Aurantiol and Macrocyclic Musks
Aurantiol's effectiveness as a fixative and floral enhancer is amplified when combined with macrocyclic musks such as ambrettolide, muscone, or habanolide. These musks, synthetic analogues of natural musk compounds, provide a clean, smooth, and subtly sweet base note.
This synergy reflects the evolution of perfumery from animal-derived fixatives like musk Tonkin and civet tincture—known for their intense, sometimes fecal character—to the modern synthetic musks developed in response to ethical and regulatory limitations. As highlighted in works by Dr. Philip Kraft and David Pybus, macrocyclic musks mimic the fixative power of natural musks without their volatility, allowing compounds like Aurantiol to bloom more slowly and elegantly in a finished fragrance.
Historically, compositions like Giorgio Beverly Hills utilized similar Schiff bases in conjunction with aldehydes and musks to create iconic, long-lasting floral signatures.
Sources
Scentspiracy Internal Archive – Schiff Base & Imines Review
David Pybus & Charles Sell (2006). The Chemistry of Fragrances
Dr. Philip Kraft in “Chemistry and Technology of Flavors and Fragrances”
IFRA Ingredient Library – Schiff Base Structures
Arctander, S. (1969). Perfume and Flavor Chemicals (Aroma Chemicals)
Peer-reviewed fragrance chemistry literature – Schiff base synthesis and applications