Javanol
Premium Synthetic Ingredient for Perfumery
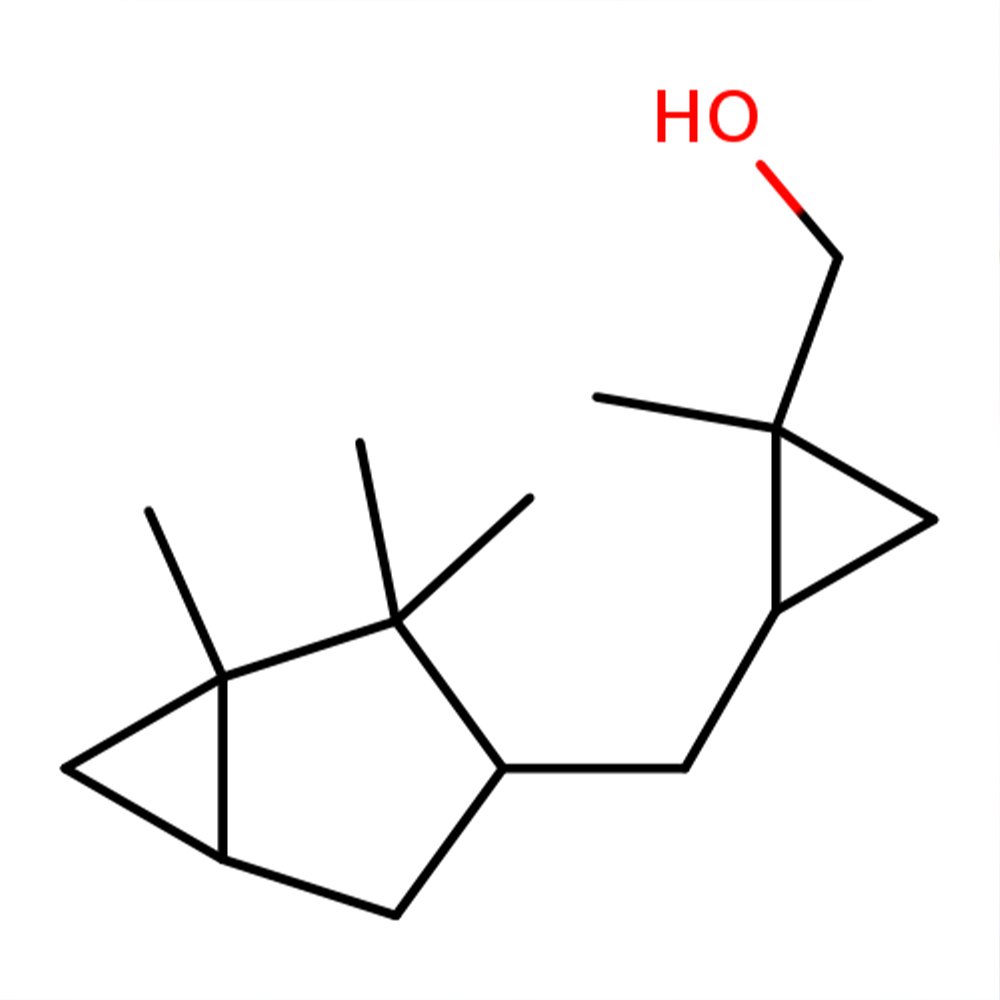
Javanol (CAS 198404-98-7) is a high-impact synthetic sandalwood molecule developed by Givaudan to emulate and exceed the performance of natural sandalwood oil. Characterized by its rich, creamy, and rosy-woody profile, Javanol offers exceptional substantivity—lasting over 400 hours on blotter—and a uniquely low odor threshold. Its molecular design, combining a rigid cyclopropyl spacer and an alcohol functional group, results in unmatched receptor binding efficiency.
Javanol is widely used across modern sandalwood bases and as a radiant fixative in musky, floral, and spicy accords.
Premium Synthetic Ingredient for Perfumery
Javanol (CAS 198404-98-7) is a high-impact synthetic sandalwood molecule developed by Givaudan to emulate and exceed the performance of natural sandalwood oil. Characterized by its rich, creamy, and rosy-woody profile, Javanol offers exceptional substantivity—lasting over 400 hours on blotter—and a uniquely low odor threshold. Its molecular design, combining a rigid cyclopropyl spacer and an alcohol functional group, results in unmatched receptor binding efficiency.
Javanol is widely used across modern sandalwood bases and as a radiant fixative in musky, floral, and spicy accords.
Premium Synthetic Ingredient for Perfumery
Javanol (CAS 198404-98-7) is a high-impact synthetic sandalwood molecule developed by Givaudan to emulate and exceed the performance of natural sandalwood oil. Characterized by its rich, creamy, and rosy-woody profile, Javanol offers exceptional substantivity—lasting over 400 hours on blotter—and a uniquely low odor threshold. Its molecular design, combining a rigid cyclopropyl spacer and an alcohol functional group, results in unmatched receptor binding efficiency.
Javanol is widely used across modern sandalwood bases and as a radiant fixative in musky, floral, and spicy accords.
Synthetic Ingredient Overview
🏭 Manufacturer: Givaudan
🔎 Chemical Name: [(1R,2S)-1-methyl-2-[[[(1R,3S,5S)-1,2,2-trimethyl-3-bicyclo[3.1.0]hexanyl]methyl]cyclopropyl]methanol
🧪 Synonyms: Javanol
🧬 Chemical Formula: C₁₅H₂₆O
📂 CAS N°: 198404-98-7
📘 FEMA: Not listed (not for flavor use)
⚖️ MW: 222.37 g/mol
📝 Odor Type: Woody (Sandalwood)
📈 Odor Strength: High; >400 hours on blotter
👃🏼 Odor Profile: Creamy sandalwood, rosy, woody, citric rose nuance; powerful and long-lasting
⚗️ Uses: Fixative, Sandalwood base, Floral/woody modifier
🧴 Appearance: Colorless to slightly viscous liquid
What is Javanol?
Javanol is a sandalwood-type odorant synthesized as part of Givaudan’s long-standing macrocyclic and alicyclic musk research program. Discovered by Jerzy Bajgrowicz in 1996, Javanol was designed to optimize olfactory receptor binding by mimicking the electronic shape and rigidity of natural β-santalol—the primary olfactory component of sandalwood oil.
The molecule's design substitutes the reactive double bond typically present in sandalwood analogs with a cyclopropane ring, which provides both steric bulk and electron density. This enhances binding to GPCR receptors in the olfactory epithelium, especially those coded for sandalwood perception. The result is a highly diffusive, ultra-long-lastingmaterial with creamy, woody warmth and a rose-like brightness.
Unlike natural oil, Javanol is not photolabile and offers excellent oxidative and thermal stability, making it suitable for most applications except highly reactive systems like bleach.
Olfactory Profile & Perfumery Applications
Javanol’s profile is complex, balancing dense creamy sandalwood with:
Rosy-floral nuances
Citrus-like top sparkle
Warm woody persistence
Fragrance performance:
One of the longest-lasting synthetic sandalwoods (400+ hours on blotter)
Highly efficient at dosages <0.1%
Weight-for-weight, ~8× more potent than comparable synthetics in laundry retention tests
Applications in perfumery:
Modern sandalwood bases (replacing or supporting natural Mysore oil)
Core of fine fragrance sandalwood structures
Creamy depth in florals (especially rose, iris, jasmine)
Woody-musky compositions (pairs well with musks, ambroxides)
Radiance enhancer in trace use (0.02–0.1%) for diffusion and volume
Examples of use:
Truth for Men (Calvin Klein)
Chic for Men (Carolina Herrera)
Givaudan’s own Sandalwood Givco base
Suggested internal link: See The Musks: An Insight for synergistic macrocyclic interactions.
Industrial & Technical Uses
While not flavor-approved, Javanol’s structural robustness allows it to perform across:
Alcohol-based perfumes (EDT, EDP, extrait)
Functional formats: soaps, creams, shampoos, body washes
Fabric care: laundry perfumes, fabric softeners, diffusion systems
Base creation: Woody, floral, amber bases requiring roundness and longevity
It is typically stable under UV, oxygen, and mild acid/base conditions, but not bleach-resistant due to alcohol functionality.
Regulatory & Safety Overview
IFRA Classification: Usage level up to 2% (20% in fragrance concentrate)
ECHA Classification:
R36/38 – Irritating to eyes and skin
Not listed as CMR or PBT
Storage/Handling:
S02 – Keep out of reach of children
S24/25 – Avoid skin/eye contact
S26 – Rinse immediately if eye contact occurs
S36 – Wear suitable protective clothing
Environmental Notes: No major concerns related to bioaccumulation or aquatic toxicity reported under normal usage.
Additional Information
Molecular innovation: Cyclopropane-based rigidity for increased receptor binding
Low odor threshold: Radiant even in sub-percentile doses
Can skew overly “rosy” if overdosed; careful modulation with lactones, ambers, or other woody materials recommended
Benchmark for modern synthetic sandalwoods—superior to older molecules like Sandela, Brahmanol, or Bacdanol in terms of strength and clarity
Not to be confused with:
Polysantol (Firmenich – sweeter, more lactonic)
Ebanol (Givaudan – softer, less creamy)
Sandalore (IFF – linear, woody-spicy)
Sources
Fulvio Ciccolo – Scentspiracy Archives
Perfumer & Flavorist Magazine – Antoine Gaillard, Givaudan (2007)
Givaudan internal literature & Javanol datasheet
TGSC Information System
PubChem Compound Summary – CID 22096564
NCBI & ECHA Substance Info