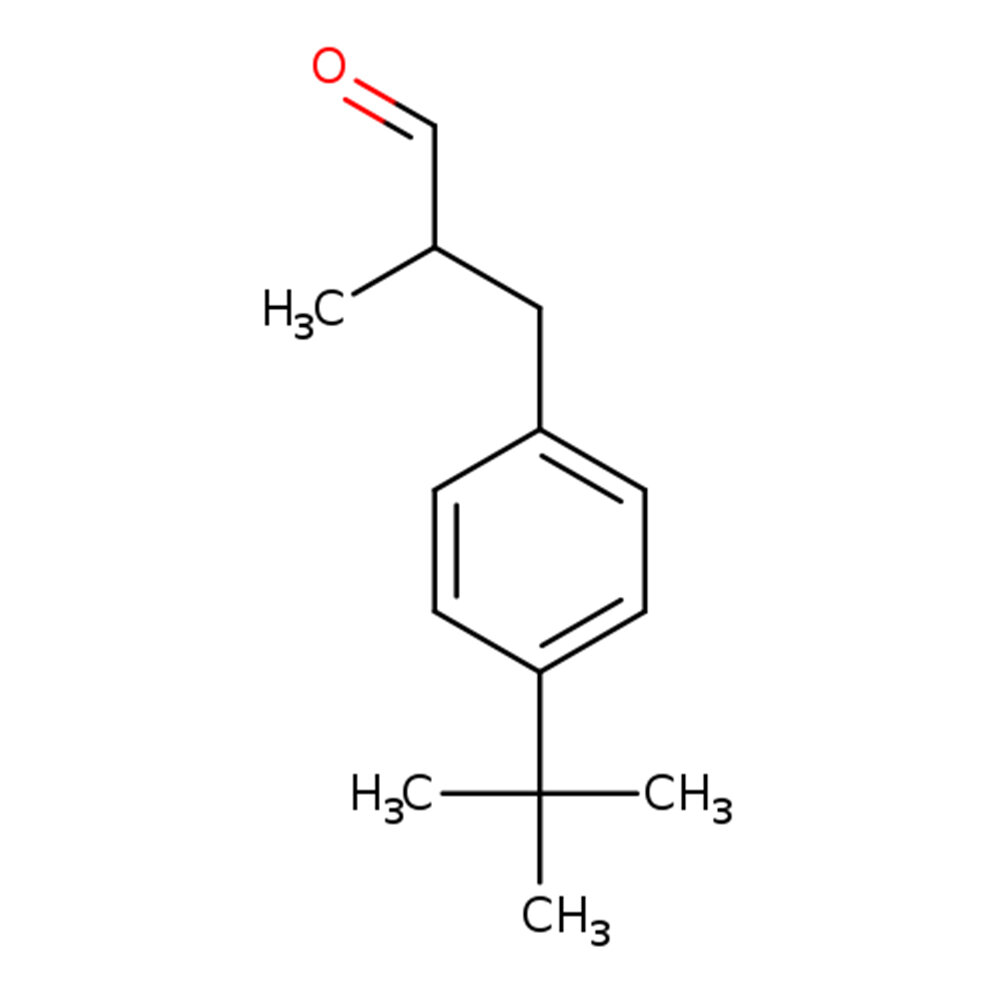
Lysmal (Lilial)
Premium Synthetic Ingredient for Perfumery
Lilial or Lysmal (CAS 80-54-6) is a synthetic aromatic aldehyde used in perfumery for its intense floral, muguet-like character. Typically sold as a racemic mixture, it exhibits high radiance and tenacity, serving as a floralizer and modifier in both fine fragrances and functional products. Only the (R)-enantiomer contributes significantly to odor; the (S)-form is essentially non-odorous. Due to toxicological concerns, it is now IFRA-restricted.
Premium Synthetic Ingredient for Perfumery
Lilial or Lysmal (CAS 80-54-6) is a synthetic aromatic aldehyde used in perfumery for its intense floral, muguet-like character. Typically sold as a racemic mixture, it exhibits high radiance and tenacity, serving as a floralizer and modifier in both fine fragrances and functional products. Only the (R)-enantiomer contributes significantly to odor; the (S)-form is essentially non-odorous. Due to toxicological concerns, it is now IFRA-restricted.
Premium Synthetic Ingredient for Perfumery
Lilial or Lysmal (CAS 80-54-6) is a synthetic aromatic aldehyde used in perfumery for its intense floral, muguet-like character. Typically sold as a racemic mixture, it exhibits high radiance and tenacity, serving as a floralizer and modifier in both fine fragrances and functional products. Only the (R)-enantiomer contributes significantly to odor; the (S)-form is essentially non-odorous. Due to toxicological concerns, it is now IFRA-restricted.
Synthetic Ingredient Overview
🔎 Chemical Name: 3-(4-tert-Butylphenyl)-2-methylpropanal
🧪 Synonyms: Butylphenyl methylpropional, Lysmeral, p-BMHCA, Lilyal
🧬 Chemical Formula: C₁₄H₂₀O
📂 CAS N°: 80-54-6 (racemate); 75166-30-2 (S), 75166-31-3 (R)
📘 FEMA: 2639
⚖️ MW: 204.31 g/mol
📝 Odor Type: Muguet, Aldehydic
📈 Odor Strength: High
👃🏼 Odor Profile: Floral, sweet, aldehydic, clean, soapy, watery, plastic, synthetic
⚗️ Uses: Floralizer, Modifier
🧴 Appearance: Colorless to pale yellow liquid
What is Lilial?
Lilial is a synthetic aromatic aldehyde, formally known as 3-(4-tert-butylphenyl)-2-methylpropanal. Structurally related to bourgeonal, it features a chiral center adjacent to the aldehyde group, yielding two enantiomers: only the (R)-enantiomer exhibits a potent floral-muguet odor; the (S)-enantiomer is virtually non-odorous. Lilial is manufactured via anodic oxidation of tert-butyltoluene and is stabilized post-production with alpha-tocopherol to prevent degradation to lysmerylic acid.
Due to keto-enol tautomerism, racemization occurs easily, making stable isolation of enantiomers impractical. Lilial is primarily distributed as a racemate.
Olfactory Profile & Perfumery Applications
Lilial is a high-impact muguet floralizer, offering aldehydic brilliance with fresh, clean, and watery nuances. It bridges floral accords with musky and woody materials and is less prone to overdose than Cyclamen Aldehyde. Although synthetic-smelling compared to Hydroxycitronellal, its tenacity and synergy compensate for cost and formulation limitations.
Common fragrance applications include:
Lily, Lilac, Muguet, Orange Blossom, Magnolia, Sweet Pea
Chypres, Musky bases, Soap perfumes
Woody and aldehydic-fantasy blends
Suggested internal link: See Smell of Specific Chemical Groups for aldehydic profiles.
Industrial & Technical Uses
Lilial has historically been included in:
Cosmetic products (creams, lotions, hair styling)
Detergents and fabric softeners
Air fresheners, deodorants, and household cleaning agents
Its cost-efficiency, radiance, and high compatibility with modern bases made it popular in both fine fragrance and mass-market perfumery until restrictions were imposed.
Regulatory & Safety Overview
Scientific Opinion (SCCS/1591/17)
The Scientific Committee on Consumer Safety (SCCS) published a 68-page assessment in 2019. Key conclusions include:
Individual product use only: Lilial (CAS 80-54-6), when stabilized with 200 ppm alpha-tocopherol, was considered safe in individual cosmetic products within defined IFRA concentration limits.
Aggregate exposure not safe: The combined use of multiple Lilial-containing products may exceed safe exposure thresholds.
Sprayable products excluded: Lilial must not be used in aerosol or spray applications due to potential inhalation toxicity.
Meta-isomer restriction: The meta-isomer (CAS 62518-65-4), classified as Repr. 1B (may damage fertility or the unborn child), must remain below 0.1%.
Lilial was later delisted from Annex III of the EU Cosmetics Regulation and prohibited under Annex II (entry 1666) in 2022. It remains listed as a known fragrance allergen.
Suggested internal link: IFRA 51st Amendment – full regulatory context.
Additional Information
Odor activity is enantioselective: only (R)-Lilial is perceptible.
Structurally related to Cyclamen Aldehyde, yet more stable in formulation.
According to Arctander (1969), Lilial is “sweet, green, and intensely floral.”
Shelf life: 2 years at 25 °C, protected from oxidation by α-tocopherol.
Molecular structure of Lilial showing its chiral center and enantiomeric forms (Scentspiracy, 2021)
Sources
User-provided source
SCCS/1591/17 Final Opinion, 2019
ECHA Substance Information (CAS 80-54-6)
Perfume and Flavor Chemicals, Steffen Arctander (1969)
FEMA GRAS Database
Zeitschrift für Lebensmitteluntersuchung – Bartschat et al., 1997
Scentspiracy Archives – Fulvio Ciccolo, 2021