 Image 1 of 2
Image 1 of 2

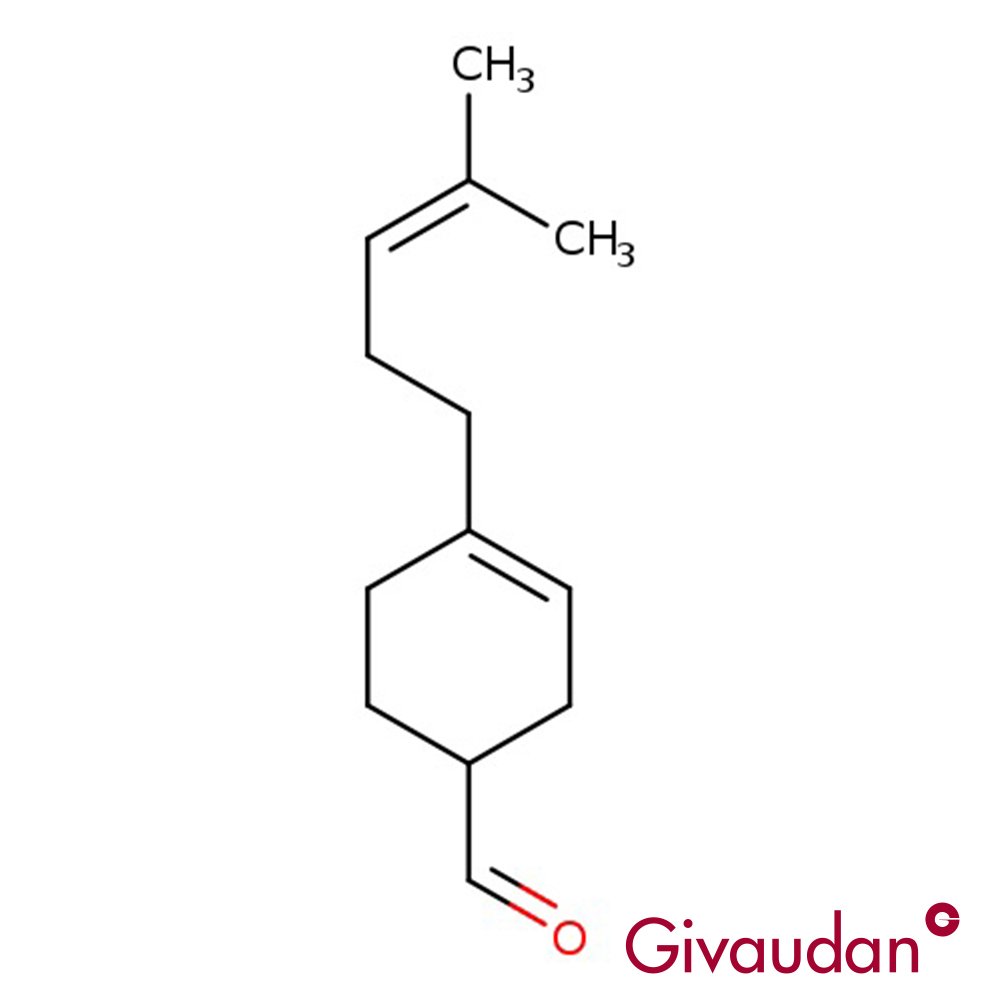
 Image 2 of 2
Image 2 of 2

Myraldene ®
Premium Synthetic Ingredient for Perfumery
Myraldene, also known as Empetal, is a synthetic aliphatic aldehyde first patented by Givaudan in the 1970s. It features a strong aldehydic profile with a waxy-citrusy opening that becomes floral and clean in dilution. Functionally, it acts as a versatile modifier and top-note enhancer, widely used in detergents, soaps, and low-cost perfumery for its penetrating brightness and aldehydic lift.
Premium Synthetic Ingredient for Perfumery
Myraldene, also known as Empetal, is a synthetic aliphatic aldehyde first patented by Givaudan in the 1970s. It features a strong aldehydic profile with a waxy-citrusy opening that becomes floral and clean in dilution. Functionally, it acts as a versatile modifier and top-note enhancer, widely used in detergents, soaps, and low-cost perfumery for its penetrating brightness and aldehydic lift.
Premium Synthetic Ingredient for Perfumery
Myraldene, also known as Empetal, is a synthetic aliphatic aldehyde first patented by Givaudan in the 1970s. It features a strong aldehydic profile with a waxy-citrusy opening that becomes floral and clean in dilution. Functionally, it acts as a versatile modifier and top-note enhancer, widely used in detergents, soaps, and low-cost perfumery for its penetrating brightness and aldehydic lift.
Synthetic Ingredient Overview
🏭 Manufacturer: Givaudan (original patent holder)
🔎 Chemical Name: 3-(4-tert-butylphenyl)-2-methylpropanal
🧪 Synonyms: Myraldene, Empetal
🧬 Chemical Formula: C13H20O
📂 CAS N°: 37677-14-8 / 52475-89-5
⚖️ MW: 192.30 g/mol
♨️ Vapor Pressure (20 °C): 0.0067 hPa
📝 Odor Type: Aldehydic
📈 Odor Strength: High (recommended in dilution); Medium tenacity
👃🏼 Odor Profile: Powerful, penetrating, waxy-citrusy; becomes fresh, clean, floral in dilution; comparable to dodecanal, cyclamal, and myristic aldehyde
👅 Flavor Profile: Waxy, slightly bitter, not pleasant
⚗️ Uses: Modifier, aldehydic booster, detergent fragrance base
🧴 Appearance: Colorless to pale yellow liquid
What is Myraldene / Empetal?
Myraldene is a non-linear, branched aliphatic aldehyde belonging to the terpenoid chemical class. Structurally related to synthetic musks and derived from substituted benzyl aldehydes, it is the chemical precursor to Lyral, though direct hydration yields poor efficiency. Its compact structure and low cost make it suitable for use in high-volume fragrance products, especially where aldehydic lift is needed without the fatty character of straight-chain aldehydes.
Olfactory Profile & Perfumery Applications
👃🏼 Odor: Sharp, aldehydic, waxy-citrusy in concentrate. Diluted, it reveals a light floral quality reminiscent of lilac and muguet.
⚗️ Functionality: Boosts brightness and aldehydic character in citrus, marine, and floral compositions.
🧴 Applications: Suitable for soap, detergent, and industrial perfumery due to its resistance to alkaline environments. Also functions well in fabric care and household cleaners.
🔗 Synergies: Performs well with ionones, cedarwood-derived cyclohexane compounds, and musky or ozonic elements in aquatic and fresh accords.
Industrial & Technical Uses
Myraldene is commonly used in:
Bar and liquid soaps
Industrial cleaners
Laundry care products
Air care and masking fragrances
Its high performance-to-cost ratio and functional freshness make it a reliable component in formulations requiring aldehydic top lift and long-lasting presence.
Regulatory & Safety Overview
IFRA: Not currently restricted; however, note its chemical proximity to Lyral (now banned under IFRA 51st Amendment)
EU Allergens: Not among the 26 listed fragrance allergens
FEMA: Not GRAS; not suitable for flavor applications
ECHA (REACH): Low vapor pressure; no current classification as sensitizer or CMR
Toxicology: No known sensitization issues at typical use levels; low oral palatability and not suitable for ingestion
Additional Information
Originally patented by Givaudan (1970s)
Serves as the immediate precursor to Lyral
Frequently used by Fulvio Ciccolo in marine and citrus-fresh accords
Enhances bergamot and top citrus notes with clean aldehydic brilliance
Sources
Fulvio Ciccolo, 2022
Perfume and Flavor Chemicals – S. Arctander, 1969
A Fragrant Introduction to Terpenoid Chemistry – Charles S. Sell
ECHA Substance Information
IFRA 51st Amendment