Allyl Caproate
Synthetic Ingredient for Perfumery
Allyl Caproate, also known as Allyl Hexanoate, is a synthetic ingredient prized in perfumery for its sweet, fruity, and ester-y pineapple aroma. This colorless liquid, often appearing yellowish in commercial samples, occurs naturally in pineapples. It’s highly valued for creating tropical and juicy fragrance profiles.
Synthetic Ingredient for Perfumery
Allyl Caproate, also known as Allyl Hexanoate, is a synthetic ingredient prized in perfumery for its sweet, fruity, and ester-y pineapple aroma. This colorless liquid, often appearing yellowish in commercial samples, occurs naturally in pineapples. It’s highly valued for creating tropical and juicy fragrance profiles.
Synthetic Ingredient for Perfumery
Allyl Caproate, also known as Allyl Hexanoate, is a synthetic ingredient prized in perfumery for its sweet, fruity, and ester-y pineapple aroma. This colorless liquid, often appearing yellowish in commercial samples, occurs naturally in pineapples. It’s highly valued for creating tropical and juicy fragrance profiles.
Technical Ingredient Overview
🔎 Chemical Name — Allyl Hexanoate
🧪 Synonyms — Allyl caproate, 3-hexenoic acid, allyl ester
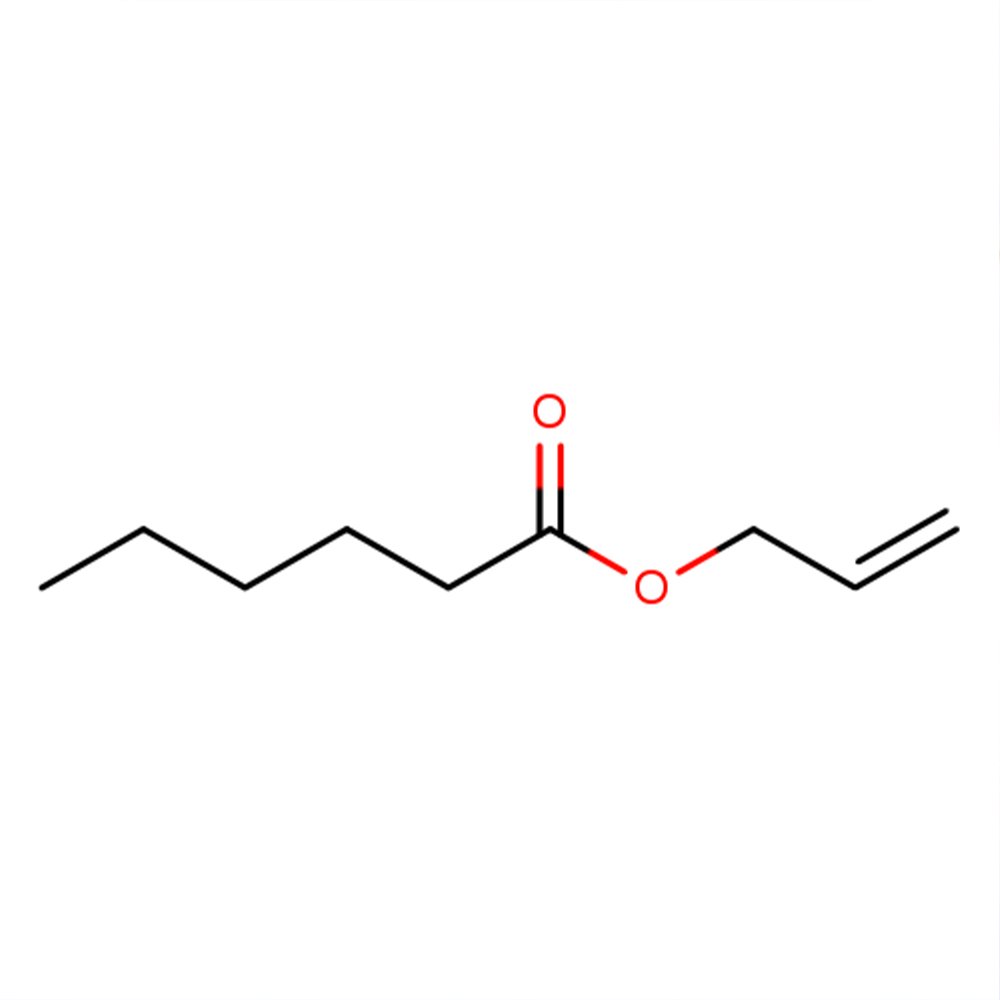
🧬 Chemical Formula — C9H16O2
📂 CAS — 123-68-2
📘 FEMA — 2032
⚖️ MW — 156.22 g/mol
📝 Odor Type — Fruity / Tropical Ester
📈 Odor Strength — High (trace use recommended)
👃🏼 Odor Profile — Sweet, fruity, ester-y, juicy, caproate-like, pineapple. sweet fruity pineapple tropical ethereal rum fatty cognac
⚗️ Uses — Allyl hexanoate is employed principally in the formulation of pineapple flavors but it can also be used for peach and apricot essences and for apple blossom, peach blossom, strawberry, and wisteria perfume compositions. Allyl caproate finds use in perfumes as a part of fruity top notes in combination with green mossy notes. It also tends to round off the Aldehyde notes in combination with styrallyl esters. lifts the top note of fruit complexes; imparts typical apple and pineapple characteristics; gives sweet fruity aspects from 0,1 to 5%. It is an ingredient of some lipstick perfumes and adds a sweet juicy note to citrus flavors. Stable in body lotion (very good), shampoo/shower gel (good), soap (good), AP roll-on 15% (good), detergent powder conc. (very good), cleaner liquid citric acid (poor), cleaner APC liquid (very good), bleach (poor). Flavor use: citrus, red fruit, yellow fruit, tropical fruit, fruit others Recommended usage up to 6%.
🧴 Appearance — Colorless to pale yellow liquid
What is Allyl Caproate?
Allyl Caproate is a synthetic fatty acid ester belonging to the allyl ester class of compounds, formed by the esterification of hexanoic acid (caproic acid) with allyl alcohol. Due to its intense and characteristic pineapple-like odor, Allyl Caproate is a powerful and widely used aroma compound in both perfumery and flavor applications, particularly for recreating tropical and exotic notes.
Personal note: I believe the allyl functional group is directly related to the pineapple aroma. We will explore this idea further.
Historical Background
The synthesis of allyl esters dates back to early 20th-century developments in organic ester chemistry, with Allyl Caproate becoming commercially significant due to its intense fruitiness and volatility. While the exact date of first synthesis is not precisely documented, esters like Allyl Caproate gained traction during the post-WWII era, coinciding with the boom in artificial flavor and fragrance materials. The molecule is commonly listed in classical perfumery references such as Arctander’s Perfume and Flavor Chemicals (1969), where it is described as one of the strongest pineapple materials available.
Olfactory Profile
Scent Family:
Fruity esters / Tropical fruits
Main Descriptors:
Pineapple
Banana
Juicy
Overripe
Slightly waxy
Intensity & Tenacity:
High impact at extremely low dosages
Very volatile and diffusive, suitable for top notes
Moderate tenacity with rapid evaporation on skin and blotter
Applications in Fine Fragrance:
In perfumery, Allyl Caproate is mainly used in trace amounts to bring realism and juiciness to fruity compositions. Its role is prominent in tropical and pineapple accords, and it is often paired with other esters (such as ethyl butyrate, methyl hexanoate) to build complexity in fruity top notes. It is also used to brighten floral-fruity bouquets and exotic formulations.
Notable accords include:
Pineapple fantasy blends
Pina colada-type creations
Fruity cocktail compositions
Headspace reconstructions of tropical fruits
Performance in Formula:
Very effective in minute concentrations (typically below 0.1%)
May become overpowering or artificial if overdosed
Blends well with lactones, other esters, ionones, and fruity aldehydes
Poor fixative properties due to high volatility, but excellent for initial impact and lift
Industrial & Technical Uses
Outside of perfumery, Allyl Caproate is extensively used in the flavor industry, particularly in fruit flavor formulationssuch as pineapple, banana, passionfruit, and exotic cocktail blends. It is FEMA GRAS approved (FEMA 2032) and regulated for food use under appropriate concentrations.
It may also serve in aroma research for recreating the volatile fractions of fresh tropical fruits and is sometimes used in functional perfumery (e.g., air care, cleaning products) where a burst of fruity freshness is desired.
Regulatory & Safety Overview
IFRA Status: Not restricted in IFRA 51st amendment; general usage must follow good manufacturing practices.
GHS Classification:
Skin Irritant (Category 2)
Eye Irritant (Category 2A)
Hazardous to aquatic life (Category 2)
EU Cosmetics Regulation (Annex III/IV): Not specifically listed; use is allowed under general safety principles.
Allergen Risk: Not a listed allergen under current EU 1223/2009 regulation.
Toxicology: LD50 (oral, rat): ~3,000 mg/kg; relatively low acute toxicity, but skin/eye irritation possible.
REACH: Registered substance under EU REACH regulations.
References
Arctander, S. (1969). Perfume and Flavor Chemicals (Aroma Chemicals). Montclair, NJ.
Surburg, H., & Panten, J. (2016). Common Fragrance and Flavor Materials: Preparation, Properties and Uses (6th ed.). Wiley-VCH.
European Chemicals Agency (ECHA). (2024). Substance information — Allyl hexanoate. Retrieved from https://echa.europa.eu/
FEMA. (2024). FEMA GRAS 2032: Allyl Caproate. Flavor and Extract Manufacturers Association. https://www.femaflavor.org
PubChem. (2024). Allyl hexanoate | CID 31241. National Center for Biotechnology Information. https://pubchem.ncbi.nlm.nih.gov/compound/Allyl-hexanoate
IFRA Standards. (2024). 51st Amendment. International Fragrance Association.