Myrcene
Premium Synthetic Ingredient for Perfumery
Myrcene (β-myrcene) is a semi-synthetic monoterpene hydrocarbon widely used in perfumery and flavor chemistry. Though originally found in the essential oils of bay, cannabis, hops, and other botanicals, commercial myrcene is typically derived via pyrolysis of β-pinene from turpentine oil. It has a sweet, balsamic, and resinous aroma with fruity-green and citrus nuances, functioning as an odor extender, fragrance intermediate, and industrial masking agent in large-scale formulations.
Premium Synthetic Ingredient for Perfumery
Myrcene (β-myrcene) is a semi-synthetic monoterpene hydrocarbon widely used in perfumery and flavor chemistry. Though originally found in the essential oils of bay, cannabis, hops, and other botanicals, commercial myrcene is typically derived via pyrolysis of β-pinene from turpentine oil. It has a sweet, balsamic, and resinous aroma with fruity-green and citrus nuances, functioning as an odor extender, fragrance intermediate, and industrial masking agent in large-scale formulations.
Premium Synthetic Ingredient for Perfumery
Myrcene (β-myrcene) is a semi-synthetic monoterpene hydrocarbon widely used in perfumery and flavor chemistry. Though originally found in the essential oils of bay, cannabis, hops, and other botanicals, commercial myrcene is typically derived via pyrolysis of β-pinene from turpentine oil. It has a sweet, balsamic, and resinous aroma with fruity-green and citrus nuances, functioning as an odor extender, fragrance intermediate, and industrial masking agent in large-scale formulations.
Synthetic Ingredient Overview
🏭 Manufacturer: Multiple producers (from turpentine sources)
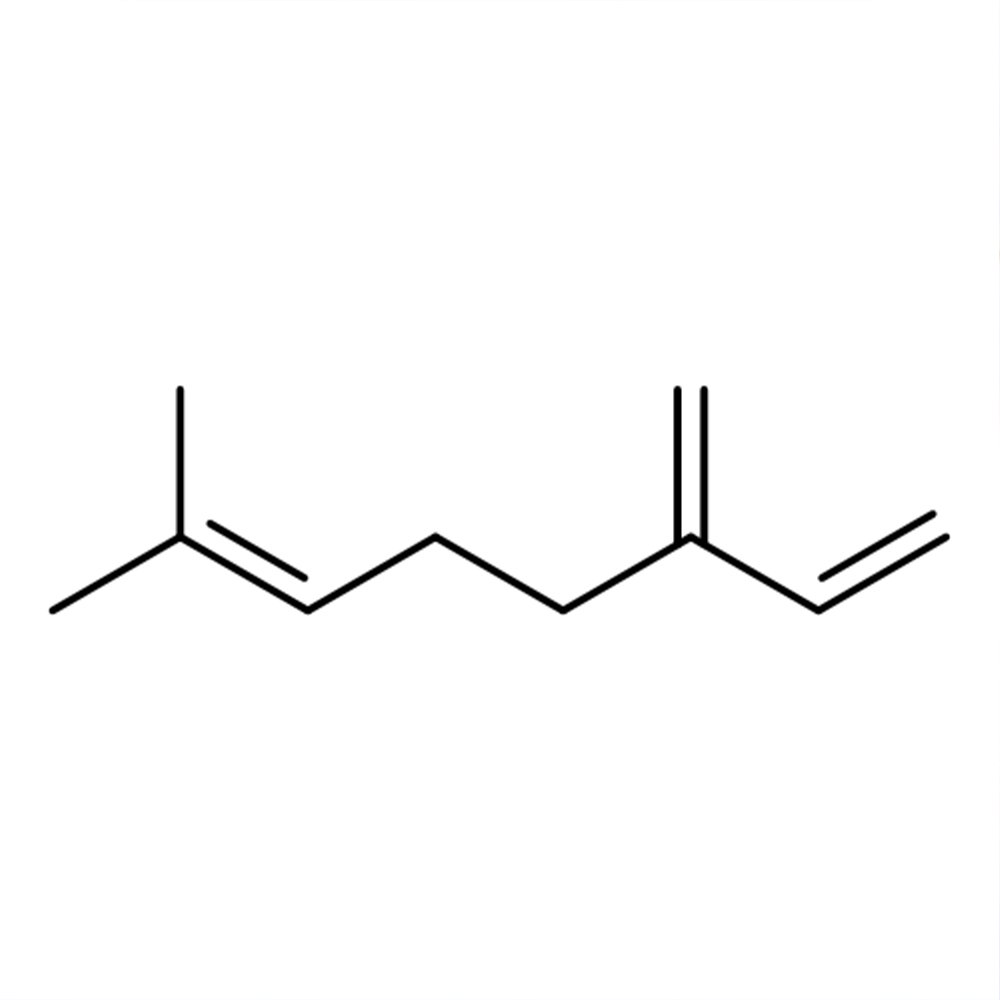
🔎 Chemical Name: 7-Methyl-3-methylene-1,6-octadiene
🧪 Synonyms: β-Myrcene, Myrca-1,5-diene
🧬 Chemical Formula: C10H16
📂 CAS N°: 123-35-3
⚖️ MW: 136.23 g/mol
📝 Odor Type: Fruity
📈 Odor Strength: Medium
👃🏼 Odor Profile: Sweet-balsamic, fruity-resinous with green mango and light citrus nuances; ethereal, slightly mushroomy, poor tenacity
👅 Flavor Profile: Sweet, balsamic-herbaceous at low levels; bitter and grassy at high concentrations
⚗️ Uses: Intermediate, Modifier, Odor Masking, Extender
🧴 Appearance: Colorless to pale yellow mobile liquid (may resinify in air)
What is Myrcene?
Myrcene is a non-cyclic monoterpene hydrocarbon that naturally occurs in several plant-derived essential oils, but is rarely extracted directly due to instability. It is classified chemically as a conjugated diene, susceptible to polymerization upon air exposure. Commercial myrcene is typically produced by pyrolyzing β-pinene at high temperatures (≈400 °C). Myrcene’s IUPAC name is 7-methyl-3-methylene-1,6-octadiene, and it functions as a key synthetic intermediate in terpene-based aroma chemistry.
Olfactory Profile & Perfumery Applications
👃🏼 Odor: Fruity, sweet-balsamic, resinous, with nuances of green mango and citrus. Fresh and volatile in its purified form, though it lacks tenacity.
⚗️ Functionality: Acts as an extender for bay leaf oil, spice colognes, and citrus accords. Contributes lightness and freshness while modifying overly sharp or fatty notes.
🧴 Applications: Suitable for colognes, deodorants, masking agents in industrial and cleaning products, and artificial essential oil formulations.
🔗 Precursors and Derivatives: Intermediate in the synthesis of geraniol, linalool, nerol, citronellal, menthol, and citral.
Industrial & Technical Uses
Myrcene is a bulk fragrance intermediate with applications in:
Industrial odor masking
Household product fragrances
Artificial essential oils
Flavor precursor chemistry
Brewing (contributes peppery, balsamic aroma in hops/beer)
Due to its volatility and air sensitivity, it is often stabilized using antioxidants like alkylphenols or tocopherol.
Regulatory & Safety Overview
IFRA: Permitted in all product categories; use levels should consider polymerization risk and odor instability
EU Allergens: Not classified among the 26 listed allergens
FEMA: FEMA No. 2762 (GRAS for flavor use)
ECHA (REACH): Classified as flammable; auto-polymerization possible under air/light exposure
Toxicology: Safe at standard industry levels; oxidation products may cause sensitization—stabilization recommended
Additional Information
Natural sources: Cannabis (29–66%), hops, bay leaf, cardamom, thyme, lemongrass
Rarely isolated directly from plants in industrial volumes
Precursor to multiple oxygenated terpenoids (alcohols, aldehydes, ketones)
Stabilized commercial forms recommended for formulation stability
Sources
National Center for Biotechnology Information (2020), PubChem CID 31253
Perfume and Flavor Chemicals – S. Arctander, 1969
ECHA Substance Information
Peer-reviewed terpenoid synthesis literature