Synthetic Ingredient Overview
🏭 Manufacturer: Historical producers: Givaudan, IFF (no longer widely produced)
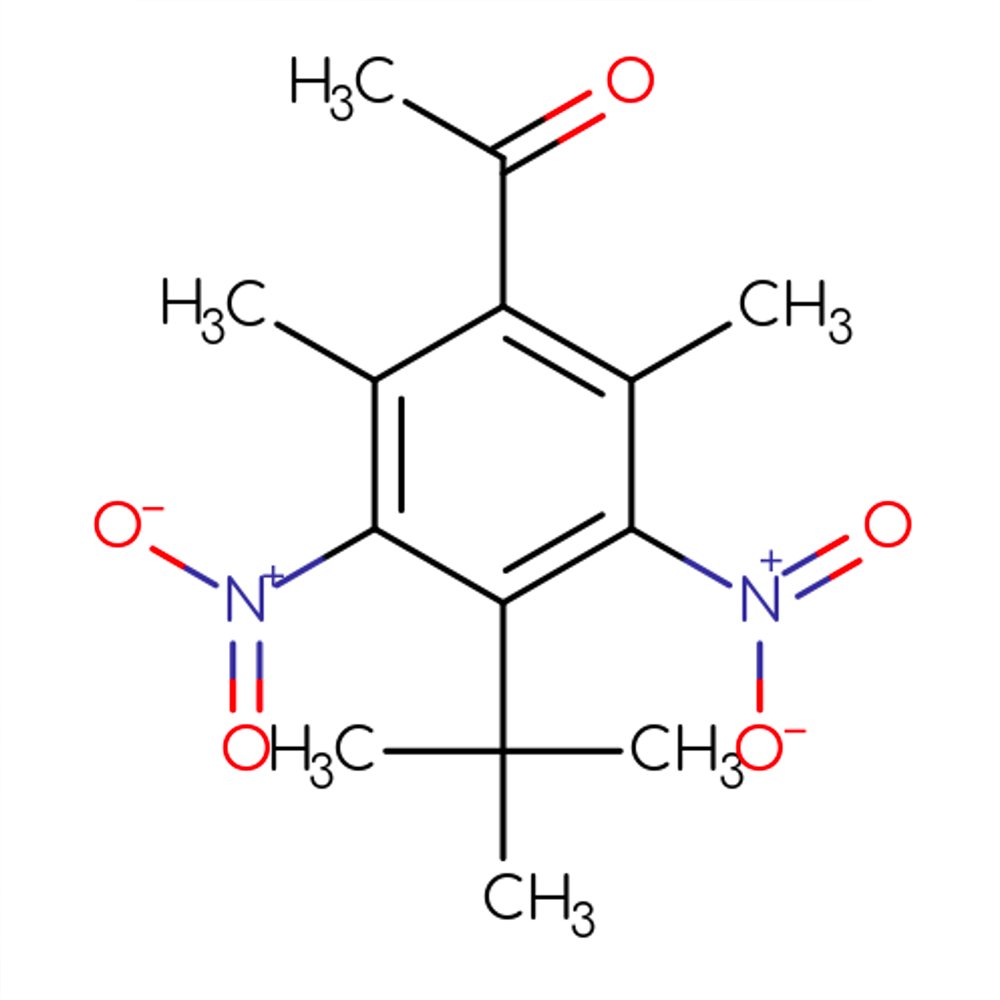
🔎 Chemical Name: 1-(4-tert-butyl-2,6-dimethyl-3,5-dinitrophenyl)ethan-1-one
🧪 Synonyms: Musk Ketone, Musk Ketonum, NSC 402029
🧬 Chemical Formula: C₁₄H₁₈N₂O₅
📂 CAS N°: 81-14-1
📘 FEMA: Not listed (not GRAS)
⚖️ MW: 294.30 g/mol
📝 Odor Type: Nitro Musk
📈 Odor Strength: High
👃🏼 Odor Profile: Sweet, musky, warm, mildly animalic, less floral than Musk Ambrette
⚗️ Uses: Fixative, Powdery-Musky Note Modifier
🧴 Appearance: Pale yellow crystalline powder
What is Musk Ketone?
Musk Ketone is a synthetic nitro musk, first developed in the early 20th century and widely used for its highly tenacious, powdery musky scent. Structurally, it is synthesized via acetylation and nitration of tert-butylxylene, forming a dinitrophenyl ketone. This gives it a persistent musky odor that remains active in formulations for extended periods.
Unlike macrocyclic musks, Musk Ketone is a nitroaromatic compound, known for its low volatility, high substantivity, and environmental persistence. Though largely phased out due to ecotoxicological and bioaccumulation concerns, it remains historically relevant and still in use under restricted conditions in some markets.
Olfactory Profile & Perfumery Applications
Musk Ketone offers a sweet, warm, and musky base note with a slightly animalic nuance. It differs from Musk Ambrette by being less floral and more linear in evolution. Its drydown is cleaner and more powdery, lending it significant utility in structuring soft, skin-like musky accords.
Key perfumery uses include:
Fixative base for powdery, floral, and oriental fragrances
Pairing with Methyl Ionones, Cinnamic Alcohol, Benzyl Salicylate to enhance sweet-floral warmth
Use in animalic-ambery or vintage musky themes
Stable performance in cold-processed soaps with minimal discoloration
Typical concentrations in fragrance concentrates range from 1% to 10%, with higher levels used in older-style bases.
Suggested internal link: See The Musks: An Insight to understand the evolution and categories of synthetic musks.
Industrial & Technical Uses
Historically, Musk Ketone was also used:
In chewing gums, candies, and beverages at concentrations of 20–45 ppm (not currently GRAS)
As a sweetener-fixative in flavor formulations (now largely discontinued)
In soaps and detergent bases for low-coloration fixative performance
However, due to regulatory status and safety reassessment, its presence in modern flavor and household fragrance systems has sharply declined.
Regulatory & Safety Overview
IFRA status: Prohibited under current IFRA guidelines (check latest amendments)
FEMA GRAS: Not listed as GRAS; formerly acknowledged by the National Research Council
EU regulation: Banned in cosmetic products (Annex II, EU Regulation 1223/2009)
Toxicology: Bioaccumulative; suspected endocrine disruptor in some models
Other notes:
May discolor under UV or in contact with amines (e.g., indole, anthranilates)
High environmental persistence and limited biodegradability
Not recommended for new formulations
Suggested internal link: Review IFRA 51st Amendment for full compliance context.
Additional Information
Often replaced by macrocyclic (e.g., Exaltolide, Ethylene Brassylate) or polycyclic musks (e.g., Galaxolide)
Still used in historical reconstructions and olfactory training for vintage fragrance development
Best stored in light-proof containers due to photodegradation sensitivity
Sources
User-provided notes
PubChem Chemistry Database
IFRA Standards Documentation
FEMA GRAS Database
Perfume and Flavor Chemicals, S. Arctander (1969)
Scentspiracy Archives