 Image 1 of 2
Image 1 of 2

 Image 2 of 2
Image 2 of 2



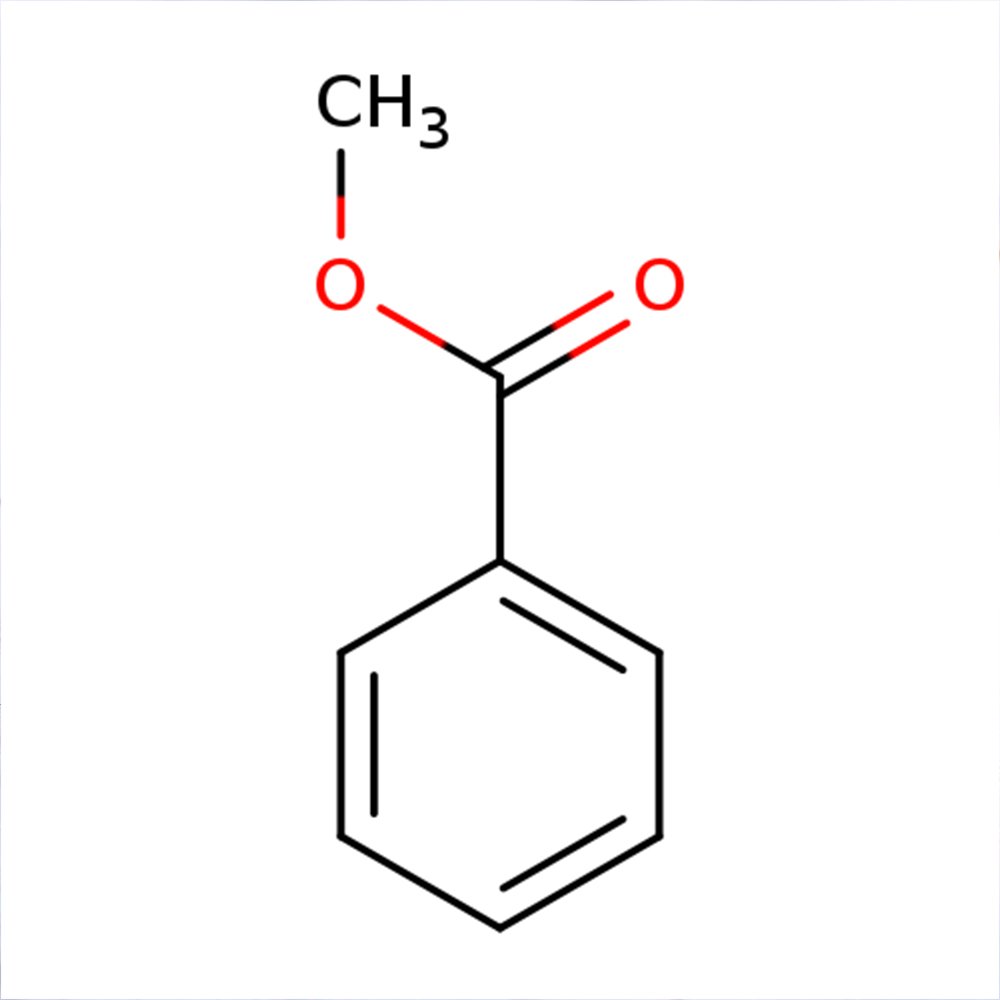
Methyl benzoate
Premium Synthetic Ingredient for Perfumery
Methyl Benzoate (CAS 93-58-3) is an aromatic ester with a powerful, narcotic-floral odor marked by phenolic and fruity overtones. Occurring in small amounts in nature (e.g., ylang-ylang, tuberose), it is primarily synthesized for use in perfumery, flavoring, entomological studies, and industrial processes. Its sharp, bitter-sweet profile finds utility in narcotic floral bases, red fruit accords, and medicinal compositions. Methyl Benzoate is also known for its use as a volatile marker in forensic scent detection and as a semiochemical in pollination ecology.
Premium Synthetic Ingredient for Perfumery
Methyl Benzoate (CAS 93-58-3) is an aromatic ester with a powerful, narcotic-floral odor marked by phenolic and fruity overtones. Occurring in small amounts in nature (e.g., ylang-ylang, tuberose), it is primarily synthesized for use in perfumery, flavoring, entomological studies, and industrial processes. Its sharp, bitter-sweet profile finds utility in narcotic floral bases, red fruit accords, and medicinal compositions. Methyl Benzoate is also known for its use as a volatile marker in forensic scent detection and as a semiochemical in pollination ecology.
Premium Synthetic Ingredient for Perfumery
Methyl Benzoate (CAS 93-58-3) is an aromatic ester with a powerful, narcotic-floral odor marked by phenolic and fruity overtones. Occurring in small amounts in nature (e.g., ylang-ylang, tuberose), it is primarily synthesized for use in perfumery, flavoring, entomological studies, and industrial processes. Its sharp, bitter-sweet profile finds utility in narcotic floral bases, red fruit accords, and medicinal compositions. Methyl Benzoate is also known for its use as a volatile marker in forensic scent detection and as a semiochemical in pollination ecology.
ynthetic Ingredient Overview
🔎 Chemical Name: Methyl benzoate
🧪 Synonyms: Benzoic acid, methyl ester; Methyl benzene carboxylate; Niobe oil; Oil of Niobe; Oxidate LE
🧬 Chemical Formula: C₈H₈O₂
📂 CAS N°: 93-58-3
📘 FEMA: 2685
⚖️ MW: 136.15 g/mol
📝 Odor Type: Narcotic, Phenolic
📈 Odor Strength: High
👃🏼 Odor Profile: Sharp, narcotic, medicinal-floral, phenolic; sweet-fruity (ylang-ylang, tuberose), slightly wintergreen
⚗️ Uses: Narcotic floral bases, red/yellow fruits, spices, solvents, bee attractant
🧴 Appearance: Colorless to pale yellow liquid
What is Methyl Benzoate?
Methyl Benzoate is an ester derived from benzoic acid and methanol, characterized by a bittersweet phenolic-floral aroma. Though naturally present in essential oils like ylang-ylang and tuberose, commercial use relies on synthetic production. In perfumery, it enhances narcotic white florals, spicy-fruity transitions, and medicinal top notes. Its behavior mimics natural components of Polianthes tuberosa and Narcissus jonquilla, making it especially valuable in soliflores and vintage floral reconstructions.
Aroma threshold: 110 ppb
Taste threshold: ~30 ppm (cherry pit, camphoraceous, balsamic)
Olfactory Profile & Perfumery Applications
The odor of methyl benzoate is:
Sharp, narcotic, and phenolic, evoking medicinal-floral notes
Sweet and fruity, with tuberose-ylang resemblance
Wintergreen-like, due to structural similarity with salicylates and cresols
Common uses:
Floral bases: Ylang-Ylang, Tuberose, Narcissus, Jonquil
Red/yellow fruit accords (cherry, plum, guava, banana)
Oriental-spicy profiles (vanilla, clove, cassia)
Fixative/volatilizer in heady and heavy florals
Used in perfume types requiring a narcotic high-floral lift, or medicinal contrast in spicy and balsamic constructions.
Molecular Synthesis & Industrial Sources
Methyl Benzoate is produced via:
Transesterification of ethyl benzoate with methanol in alkaline medium (KOH)
Direct esterification of benzoic acid with methanol, using acid catalysts
Earlier processes used dimethyl sulfate with benzoic acid at elevated temperatures
In industry, it is also a by-product in terylene (PET) manufacturing, making it more accessible through petrochemical refinement rather than dedicated synthesis.
Due to this abundance, classical lab-scale methods have largely been replaced by industrial recovery.
Ecological and Ethological Relevance
Methyl Benzoate is a semiochemically active compound, especially notable for:
Attracting male orchid bees (Euglossini) who use it to synthesize sex pheromones. It is a standard bait chemicalin entomological field studies.
Being a by-product of cocaine hydrolysis, forming in humid air—this makes it a key odorant used to train drug detection dogs. The sharp medicinal odor is highly detectable even at trace levels.
Its role in pollination ecology and forensic detection makes it unique among perfumery ingredients, bridging biological relevance and functional fragrance design.
Regulatory & Safety Overview
IFRA Classification: Generally permitted
FEMA GRAS: 2685
REACH/ECHA: Registered; not classified as hazardous
Toxicology Notes:
Low toxicity in standard use
May cause mucous membrane irritation in concentrated form
Avoid ingestion or inhalation of high vapor concentrations
Storage: Flammable; store in well-ventilated, cool area
Additional Information
Natural occurrence confirmed in:
Tuberose, ylang-ylang, clove, narcissus, banana, cherry, guava, papaya, pineapple, tea, vanilla, coffee, honey, herbs, Gruyère cheese, and countless fruits and spices.
Flavor profile:
Phenolic, medicinal, balsamic; slight cherry-pit nuance
Used in trace amounts for fruit reconstitution, spice warmth, or balsamic accents
Annual consumption: ~1300 lbs
Detected in: Tea, honey, prune, vinegar, berries, starfruit, mushrooms, olive oil, clove bud oil, cassia, basil, chamomile, dried bonito, soursop, myrtle berry, pepper, yogurt, hops.
Sources
Fulvio Ciccolo – Scentspiracy Archive
PubChem Compound Summary – CID 7150
Common Fragrance and Flavor Materials – Bauer, Garbe, Surburg (Wiley-VCH, 2001)
Fenaroli’s Handbook of Flavor Ingredients – Burdock, 6th ed.
NCBI Chemistry Database – National Center for Biotechnology Information