Menthol
Premium Synthetic Ingredient for Perfumery
Menthol (CAS 2216-51-5) is a monocyclic terpene alcohol derived from natural mint oils or produced synthetically. It is solid at room temperature, forming crystalline needles with a penetrating minty scent and distinct cooling effect. Menthol is used as a top-note booster, freshness enhancer, and functional cooling agent in perfumes, flavors, cosmetics, and therapeutic applications.
Its olfactory and sensory impact is mediated by simultaneous activation of olfactory receptors and thermosensitive ion channels.
Premium Synthetic Ingredient for Perfumery
Menthol (CAS 2216-51-5) is a monocyclic terpene alcohol derived from natural mint oils or produced synthetically. It is solid at room temperature, forming crystalline needles with a penetrating minty scent and distinct cooling effect. Menthol is used as a top-note booster, freshness enhancer, and functional cooling agent in perfumes, flavors, cosmetics, and therapeutic applications.
Its olfactory and sensory impact is mediated by simultaneous activation of olfactory receptors and thermosensitive ion channels.
Premium Synthetic Ingredient for Perfumery
Menthol (CAS 2216-51-5) is a monocyclic terpene alcohol derived from natural mint oils or produced synthetically. It is solid at room temperature, forming crystalline needles with a penetrating minty scent and distinct cooling effect. Menthol is used as a top-note booster, freshness enhancer, and functional cooling agent in perfumes, flavors, cosmetics, and therapeutic applications.
Its olfactory and sensory impact is mediated by simultaneous activation of olfactory receptors and thermosensitive ion channels.
Synthetic Ingredient Overview
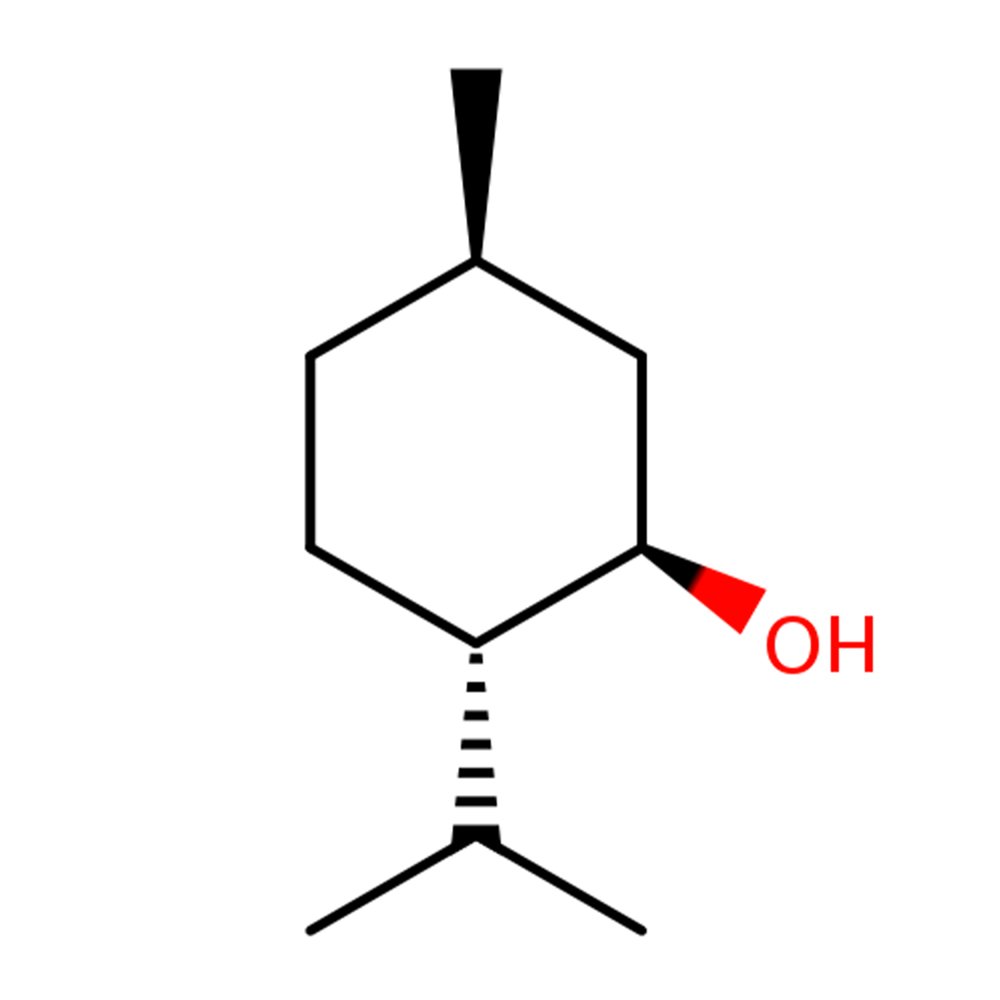
🔎 Chemical Name: 2-Isopropyl-5-methylcyclohexanol
🧪 Synonyms: Menthol, (–)-Menthol, dl-Menthol (racemic)
🧬 Chemical Formula: C₁₀H₂₀O
📂 CAS N°: 2216-51-5
📘 FEMA: 2665
⚖️ MW: 156.26 g/mol
📝 Odor Type: Fresh, Minty
📈 Odor Strength: High
👃🏼 Odor Profile: Peppermint-like, sweet, diffusive, pungent, cooling without menthone harshness
⚗️ Uses: Cooling modifier, top note booster, flavoring agent, TRPM8 activator
🧴 Appearance: White crystalline solid; melts ~36–38 °C
What is Menthol?
Menthol is a mint-sourced or synthetically produced terpene alcohol found predominantly as the (–)-enantiomer in nature. It crystallizes from mint oils such as Mentha arvensis or can be synthesized via hydrogenation of thymol, citronellal, or pulegone intermediates. Commercial grades may be enantiomerically pure or racemic depending on production route.
Its olfactory perception is tightly coupled with its somatosensory cooling effect, though the two thresholds differ—menthol may be smelled at sub-ppm levels, while its TRPM8 receptor-mediated cold sensation occurs at higher concentrations. Menthol is structurally stabilized by a chair conformation, allowing its hydroxyl, methyl, and isopropyl groups to adopt equatorial positions, enhancing physical and sensory stability.
Biological Properties of Menthol
Menthol exhibits diverse biological activity beyond its olfactory and flavoring functions. It acts as a selective agonist of the TRPM8 ion channel, a cold-sensing receptor present in sensory neurons. Activation of TRPM8 by menthol triggers the familiar cooling sensation without an actual decrease in temperature, making it valuable in topical analgesic and cosmetic applications. Menthol is also a weak κ-opioid receptor agonist, contributing to its analgesic effects, and a GABA<sub>A</sub> receptor positive allosteric modulator, which imparts mild sedative and anesthetic properties similar to propofol. Furthermore, menthol blocks voltage-gated sodium channels, decreasing neuronal excitability and enhancing its use in localized pain relief. In oral care, menthol demonstrates antibacterial activity against streptococci and lactobacilli, supporting its role in preventing dental plaque and oral biofilm formation.
Menthol Production Methods
Menthol can be obtained by both natural extraction and synthetic synthesis. The natural form is typically isolated from Mentha arvensis or Mentha piperita oils through steam distillation, followed by fractional crystallization at low temperatures. The menthone-rich residual fraction can be further hydrogenated to yield additional menthol. Synthetic production routes involve hydrogenation of thymol, pulegone, or isopulegol, yielding either racemic menthol or specific stereoisomers depending on the reaction pathway. Another industrial method derives menthol from citronellal via isopulegol intermediates. While natural menthol remains dominant in Asian markets, synthetic menthol accounts for a growing share of global production, offering higher stereoisomeric control and cost efficiency. Most commercial menthol is sold as the (–)-enantiomer, although racemic mixtures are used in technical applications.
Olfactory Profile & Perfumery Applications
Menthol imparts a diffusive, clean minty freshness, with a secondary sweetness and a mild pungency. It is widely used in:
Fougères and Aromatic Florals – enhancing lavender, geranium, rose
Soaps and toiletries – lending clean, hygienic freshness
Functional perfumery – e.g., shaving products, aftershaves
Mint accords – spearmint, peppermint, menthone systems
Typical concentrations in perfume:
0.5%–2.5% in concentrate for freshening and boosting
Used to lift and cool heavy florals or medicinal blends
Flavor applications include chewing gum, mint blends, caramel, licorice, imitation butter, fruit complexes.
Suggested internal link: See Smell of Specific Chemical Groups for terpene-based cooling and mint profiles.
Molecular Behavior & Pharmacological Properties
Menthol’s physiological and pharmacological behavior is linked to its interaction with TRPM8 cold receptors, which respond to moderate cooling stimuli. When inhaled, consumed, or topically applied, menthol activates cold-sensitive ion channels, triggering a sensation of coolness without temperature change. This makes it distinct among aroma compounds for its dual sensory-olfactory impact.
Menthol also acts as:
A κ-opioid receptor agonist, providing mild analgesia
A GABA<sub>A</sub> positive allosteric modulator, contributing to mild sedative and anesthetic effects(mechanism shared with propofol)
A voltage-gated sodium channel blocker, which inhibits nerve impulse transmission in topical analgesics
Topical menthol is used in rubefacient creams, yet it does not reduce inflammation directly. Additionally, it shows topical antibacterial activity against Streptococcus and Lactobacillus strains, contributing to its long-standing use in dental care.
Crystallographically, menthol exists in multiple polymorphs, with the α-form being the most stable and familiar—broad, needle-like crystals with a melting point near 38 °C.
Industrial & Technical Uses
Menthol is used across multiple industries:
Perfumery:
Freshening agent in personal care and functional fragrance
Booster in floral-mint, fougère, and soap perfumes
Adds cooling contrast in hot-spice and camphoraceous accords
Flavor:
Mint (peppermint/spearmint) fortifier
Used in traces for fruit, caramel, licorice, and imitation butter
Max typical concentration:
35–400 ppm (general food)
1000–1200 ppm (chewing gum, dentifrice)
Cosmetics & Therapeutics:
Shaving creams, aftershaves, cooling gels
Topical analgesics and skin-relief formulations
Oral hygiene products: toothpaste, mouthwash
Carrier compound for over-the-counter muscle rubs
Regulatory & Safety Overview
FEMA GRAS: 2665
IFRA-compliant: Generally permitted within product-specific limits
ECHA REACH Status: Not classified as hazardous under normal conditions
Toxicology:
Non-carcinogenic, non-mutagenic
Potential irritant at high concentrations
Cooling effect may cause mucous membrane discomfort
Storage advice: Store in sealed containers, below melting point, in cool/dry environments. Flammable in vapor form; standard safety protocols apply.
Sources
Perfume and Flavor Chemicals II – Steffen Arctander
PubChem Compound Summary – CID 16666
Wikipedia – Menthol Entry
FEMA GRAS Database
NCBI Scientific Literature
Scentspiracy Archives