 Image 1 of 2
Image 1 of 2

 Image 2 of 2
Image 2 of 2



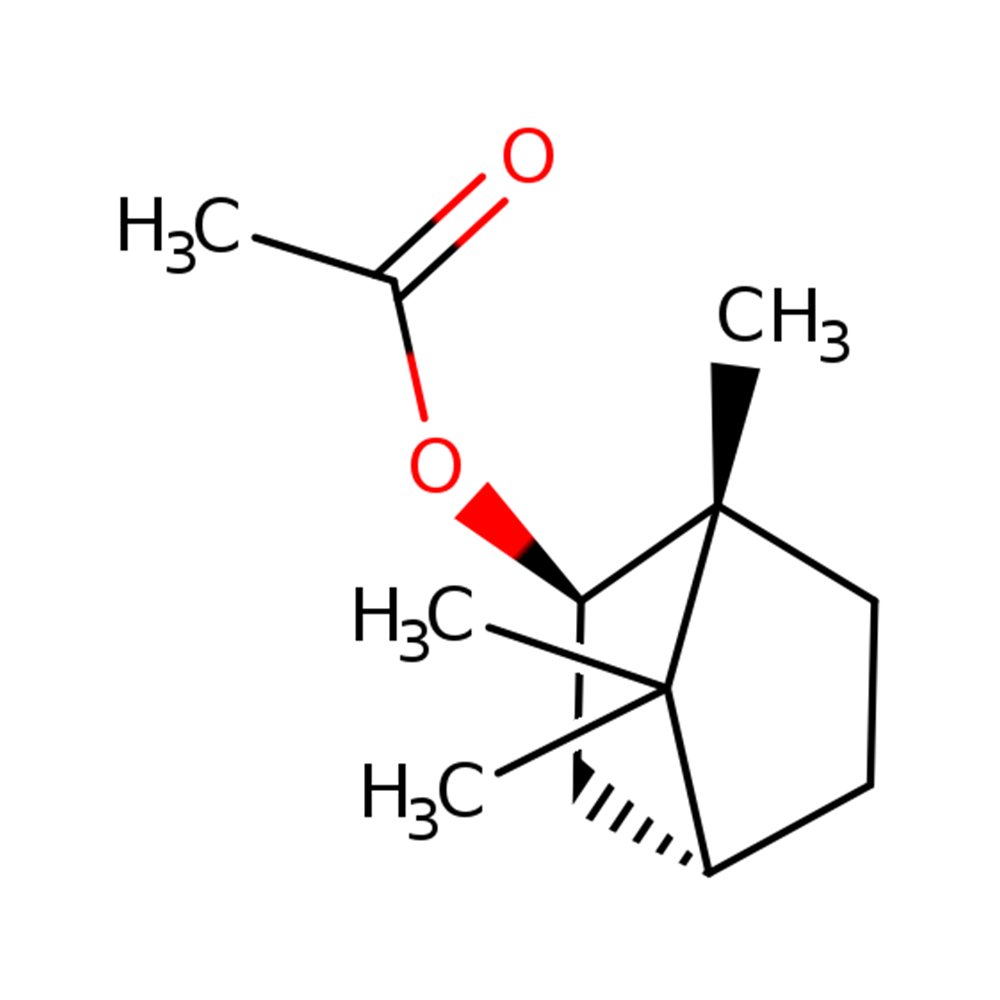
Isobornyl Acetate
Premium Synthetic Ingredient for Perfumery
Isobornyl Acetate (CAS 125-12-2) is a synthetic ester derived from camphor-type terpenes, widely recognized for its fresh, dry, woody-camphoraceous aroma and its utility in constructing herbal-green and fougère accords. Its moderate volatility and excellent blending behavior make it highly adaptable across fragrance types—from forest-themed top notes to dry, pine-resinous base enhancements. Naturally present in trace amounts in oils such as fir, basil, and rosemary, it is produced synthetically for consistent olfactory quality and high purity. Isobornyl Acetate also holds recognized flavor status and functional uses beyond perfumery.
Premium Synthetic Ingredient for Perfumery
Isobornyl Acetate (CAS 125-12-2) is a synthetic ester derived from camphor-type terpenes, widely recognized for its fresh, dry, woody-camphoraceous aroma and its utility in constructing herbal-green and fougère accords. Its moderate volatility and excellent blending behavior make it highly adaptable across fragrance types—from forest-themed top notes to dry, pine-resinous base enhancements. Naturally present in trace amounts in oils such as fir, basil, and rosemary, it is produced synthetically for consistent olfactory quality and high purity. Isobornyl Acetate also holds recognized flavor status and functional uses beyond perfumery.
Premium Synthetic Ingredient for Perfumery
Isobornyl Acetate (CAS 125-12-2) is a synthetic ester derived from camphor-type terpenes, widely recognized for its fresh, dry, woody-camphoraceous aroma and its utility in constructing herbal-green and fougère accords. Its moderate volatility and excellent blending behavior make it highly adaptable across fragrance types—from forest-themed top notes to dry, pine-resinous base enhancements. Naturally present in trace amounts in oils such as fir, basil, and rosemary, it is produced synthetically for consistent olfactory quality and high purity. Isobornyl Acetate also holds recognized flavor status and functional uses beyond perfumery.
Synthetic Ingredient Overview
🔎 Chemical Name: Bornan-2-yl acetate
🧪 Synonyms: Isobornyl acetate, 2-Bornyl acetate, Borneyl acetate
🧬 Chemical Formula: C₁₂H₂₀O₂
📂 CAS N°: 125-12-2
📘 FEMA: 2135
⚖️ MW: 196.29 g/mol
📝 Odor Type: Fresh, Terpenic
📈 Odor Strength: Medium (approx. 8 hours on strip when undiluted)
👃🏼 Odor Profile: Fresh, woody, dry-amber; reminiscent of borneol with green pine and camphoraceous nuances
⚗️ Uses: Modifier, Herbalizer, Fougère base construction
🧴 Appearance: Colorless to pale straw yellow liquid
What is Isobornyl Acetate?
Isobornyl Acetate is a terpenic ester with a bicyclic structure derived from borneol or camphene, used primarily for its dry, woody freshness and ability to create or support green, resinous, and forest-like effects in perfumery. Its synthesis is typically carried out through:
Acetylation of isoborneol, or
Catalytic addition of acetic acid to camphene, using strong acid catalysts.
The result is an optically inactive, moderately volatile compound, prized for its blending power, diffusivity, and dry clarity. It offers a technical balance between volatility and substantivity, making it a practical material in both fine fragrance and detergent formulations.
Olfactory Profile & Perfumery Applications
Isobornyl Acetate delivers a crisp, forest-like scent, suggestive of:
Pine needles
Dry conifer wood
Clean camphoraceous air
Slight dry amber and faint citrus-herbal lift
While sometimes associated with "cleaning product" freshness, in skilled formulation it lends dimensional freshness and structural support to otherwise floral or green-dominated fragrances.
Functional applications in perfumery:
Primary role in pine and conifer accords
Fougère construction, in combination with:
Coumarin (warm tonality)
Amyl Salicylate (soft floral lift)
Terpinyl Acetate or Nopyl Acetate (diffusive brightness)
Top note brightener for rosemary, sage, lavandin, artemisia
Modifier in soapy-green aldehydic fragrances or masculine florals
Comparative note: Unlike α-terpineol or terpinen-4-ol, Isobornyl Acetate does not skew overly citrusy or phenolic; its dryness provides a firm structural base to build forest impressions.
Industrial & Technical Uses
Beyond fine fragrance, Isobornyl Acetate is extensively used in:
Soap and bath fragrance bases (stable at high pH)
Shampoos and herbal hair products
Pine and herbal household products (cleansers, air fresheners)
Functional Fougères in shaving foams and deodorants
Camphor precursor (intermediate in synthetic camphor production)
Additionally, it demonstrates mild antimicrobial activity, making it of interest in preservative-free and hygiene-forward product development.
Regulatory & Safety Overview
IFRA Category 4 (leave-on skin): Safe up to 30% in fragrance concentrate
FEMA GRAS (No. 2135): Approved for use in food and beverages
Max CoE limits: 5 ppm in food, 0.1 ppm in beverages
US FDA (21 CFR 172.515): Approved as flavoring
ECHA REACH Status: Not classified as hazardous
Toxicology:
Non-toxic in typical concentrations
May cause irritation in pure form
Flammable: proper storage in cool, well-ventilated area required
No allergenic concern has been identified under standard usage conditions. Compatible with essential oil systems and synthetic compositions.
Suggested internal link: IFRA Limits for category-specific usage guidance.
Additional Information
Found naturally in oils of fir, pine, basil, thyme, and rosemary (minor component)
Offers olfactory roundness and structure to unbalanced herbal-green themes
Useful in early-stage development of neo-chypre and neo-fougère accords
Low polarity makes it soluble in oils, alcohols, and hydrocarbons, but not in water or glycerol
Flavor use: Aromatic agent in bakery, confectionery, puddings, and frozen dairy; total estimated consumption remains low globally, making it suitable for trace-use food applications.
Sources
Arctander, S. (1961). Perfume and Flavor Materials of Natural Origin
Burdock, G.A. (2010). Fenaroli’s Handbook of Flavor Ingredients, 6th ed.
ChemicalBook – Isobornyl Acetate Properties and Safety Overview
PubChem Compound Summary – CID 11558
FEMA GRAS Database – 2135
Scentspiracy Archives and Technical Database