Indol
Premium Synthetic Ingredient for Perfumery
Indole (CAS 120-72-9) is a high-impact, animalic-aromatic molecule with narcotic floral properties and profound importance in jasmine, orange blossom, and tuberose accords. Despite its harsh fecal character at high concentration, it delivers unmistakable radiance and floral diffusion in trace amounts. Common in both niche and functional perfumery, it remains a foundational element in high-diffusion floral bases and exotic compositions.
Premium Synthetic Ingredient for Perfumery
Indole (CAS 120-72-9) is a high-impact, animalic-aromatic molecule with narcotic floral properties and profound importance in jasmine, orange blossom, and tuberose accords. Despite its harsh fecal character at high concentration, it delivers unmistakable radiance and floral diffusion in trace amounts. Common in both niche and functional perfumery, it remains a foundational element in high-diffusion floral bases and exotic compositions.
Premium Synthetic Ingredient for Perfumery
Indole (CAS 120-72-9) is a high-impact, animalic-aromatic molecule with narcotic floral properties and profound importance in jasmine, orange blossom, and tuberose accords. Despite its harsh fecal character at high concentration, it delivers unmistakable radiance and floral diffusion in trace amounts. Common in both niche and functional perfumery, it remains a foundational element in high-diffusion floral bases and exotic compositions.
Synthetic Ingredient Overview
🏭 Manufacturer: Givaudan
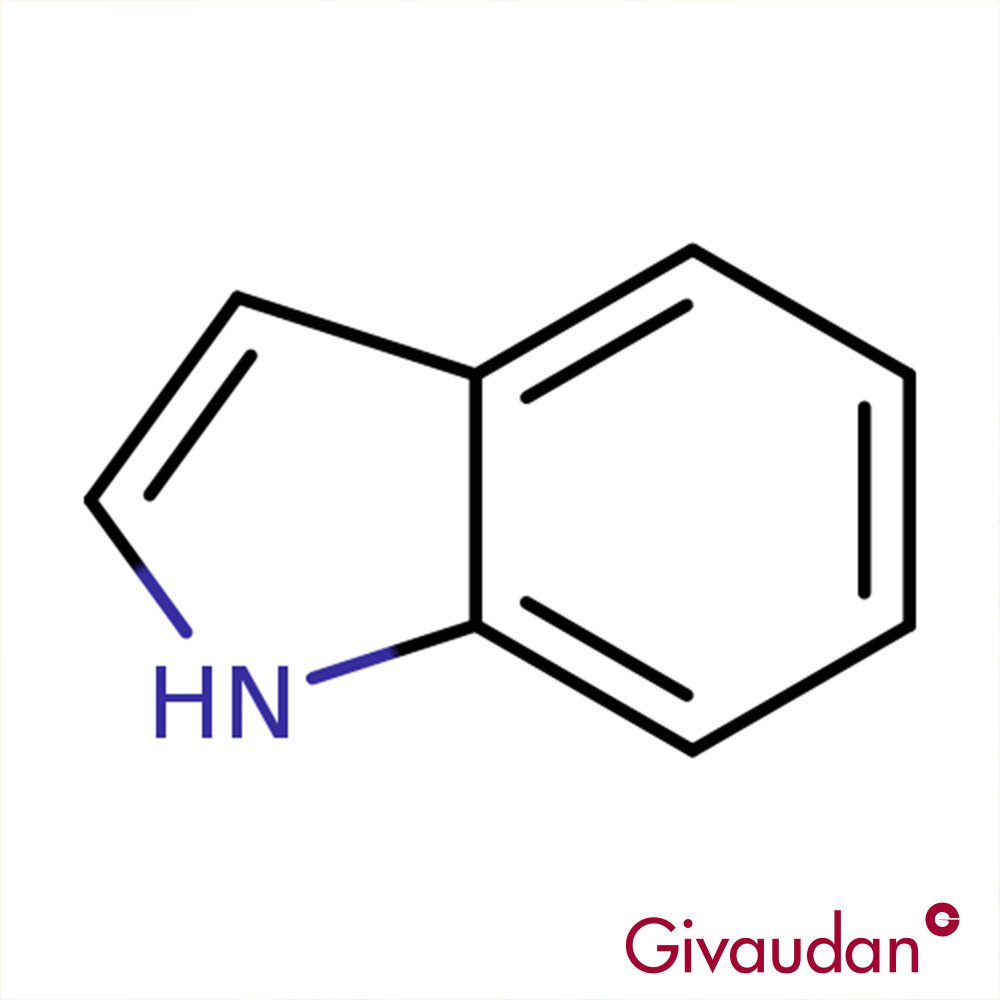
🔎 Chemical Name: Indole
🧪 Synonyms: Benzopyrrole
🧬 Chemical Formula: C₈H₇N
📂 CAS N°: 120-72-9
📘 FEMA: 2597
⚖️ MW: 117.15 g/mol
📝 Odor Type: Narcotic (Animalic / Floral)
📈 Odor Strength: High
👃🏼 Odor Profile: Pungent, floral, mothball-like with fecal-animalic character and naphthalene notes
⚗️ Uses: Floralizer for Jasmine, Tuberose, Orange Flower, and heavy-floral accords
🧴 Appearance: White to beige solid, soluble in alcohol and glycols, slightly soluble in hot water
What is Indole?
Indole is an aromatic heterocyclic organic compound with a bicyclic structure—benzene fused to pyrrole. Naturally found in jasmine, orange flower, human feces, and coal tar, indole has historically served as a foundational building block in both perfume and dye chemistry. The compound occurs naturally via microbial metabolism and also forms in roasted and aged organic matter.
It is notable for being the parent structure of tryptophan and many alkaloids, as well as a precursor in serotonin biosynthesis. Indole has been a component of perfumery since the early 20th century and was synthetically isolated via the reduction of oxindole by Adolf von Baeyer in 1866.
Olfactory Profile & Perfumery Applications
Despite its reputation for being repulsive in isolation, indole is a powerhouse fixative and modifier at ultra-low concentrations (<0.1%). When properly diluted, it enriches floral blends with realism and radiance—transforming jasmine, neroli, orange blossom, tuberose, and narcissus into rich, narcotic florals.
It pairs particularly well with:
Jasmine absolutes and synthetic jasmonates
Civet notes and musk ambrette replacements
Phenolic, naphthalene-rich bases in orientals and night-blooming floral types
Its volatility does not prevent its high tenacity; on blotters, the odor may last for weeks. In soaps, indole requires careful use due to potential discoloration and oxidation unless properly stabilized.
Industrial Synthesis of Indole
Indole can be produced via multiple synthesis routes:
Vapor-Phase Aniline Route
Reaction of substituted aniline with ethylene glycol in the presence of catalysts (200–500 °C) yields substituted indoles.
2. Leimgruber–Batcho Synthesis
Begins with o-nitrotoluene derivatives, forms intermediates via methylene bridge introduction, followed by reduction using Raney-Nickel and hydrazine.
Leimgruber–Batcho indole synthesis
3. Fischer Indole Synthesis
Condensation of phenylhydrazines with aldehydes/ketones under acidic conditions. Useful for 2- or 3-substituted indoles.
Fischer indole synthesis
These routes allow functional group control and cost-effective industrial-scale production, with applications in pharma, fragrance, and agrochemical development.
Stability, Reactivity & Formulation Challenges
Stability Issues: Indole can discolor upon exposure to aldehydes (e.g., heliotropin, hydroxyphenylbutanone), metal ions (Fe³⁺), and light. Sensitive to oxidation and condensation.
Solubility: Low solubility in water, better in alcohol, propylene glycol, diethyl phthalate.
Reactivity: Must be carefully handled in white soap formulations to prevent browning. Suggested use: add at final dilution stage, preferably in solution form.
Storage Advice: Avoid direct contact with metals and air; use sealed amber glass or lined drums.
Regulatory & Safety Overview
IFRA: Indole is permitted with caution, subject to concentration limits. Typically used below 0.1% in finished formulations.
EU 1223/2009: Not one of the 26 mandatory declarable allergens, but may react with other components.
ECHA Classification: Irritant (Category 2 Eye & Skin); not classified as carcinogenic.
REACH: Registered.
Handling: Use in dilution; solid indole crystals should be avoided in lab environments due to lingering odor contamination.
✅ Conclusion: Indole remains irreplaceable for jasmine-type floral elevation, despite requiring skillful formulation handling.
Additional Information
Perfumery Tip: For beginners or inexperienced perfumers, indole should always be introduced via pre-diluted solutions (typically 10% in DEP).
Alternative Materials: Skatole (less floral), 3-methylindole (stronger fecal), indoline (more stable).
Historical Formulas: Used in classic florals like Chanel No. 5, L’Air du Temps, and Joy.
Sources
S. Arctander, Perfume and Flavor Chemicals
Leffingwell Aroma Database
PubChem Compound Summary CID 798
The Chemistry of Fragrance
FEMS Microbiol Rev, Lee & Lee, 2010
Ullmann’s Encyclopedia of Industrial Chemistry
NCBI / PubChem