 Image 1 of 2
Image 1 of 2

 Image 2 of 2
Image 2 of 2



Hydroxycitronellal
Premium Synthetic Ingredient for Perfumery
Hydroxycitronellal is a synthetic floral aldehyde widely used for its sweet, delicate muguet (lily of the valley) profile. Introduced commercially in the early 20th century, it remains a foundational material in floral perfumery. Its medium odor strength, good tenacity, and synergy with low-boiling modifiers make it ideal for muguet, peony, and white floral compositions. Despite newer substitutes, it remains irreplaceable in numerous applications.
Premium Synthetic Ingredient for Perfumery
Hydroxycitronellal is a synthetic floral aldehyde widely used for its sweet, delicate muguet (lily of the valley) profile. Introduced commercially in the early 20th century, it remains a foundational material in floral perfumery. Its medium odor strength, good tenacity, and synergy with low-boiling modifiers make it ideal for muguet, peony, and white floral compositions. Despite newer substitutes, it remains irreplaceable in numerous applications.
Premium Synthetic Ingredient for Perfumery
Hydroxycitronellal is a synthetic floral aldehyde widely used for its sweet, delicate muguet (lily of the valley) profile. Introduced commercially in the early 20th century, it remains a foundational material in floral perfumery. Its medium odor strength, good tenacity, and synergy with low-boiling modifiers make it ideal for muguet, peony, and white floral compositions. Despite newer substitutes, it remains irreplaceable in numerous applications.
Synthetic Ingredient Overview
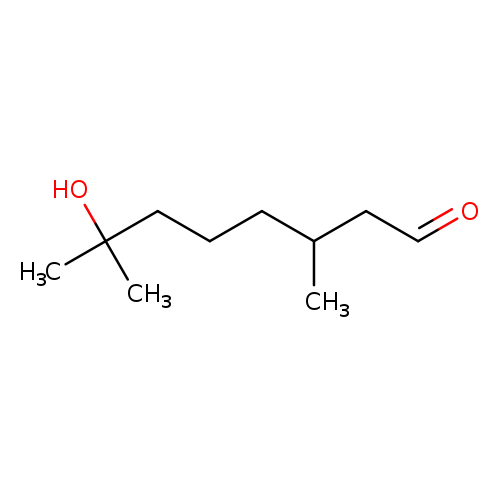
🔎 Chemical Name: 7-Hydroxy-3,7-dimethyloctanal
🧪 Synonyms: Hydroxycitronellal
🧬 Chemical Formula: C₁₀H₂₀O₂
📂 CAS N°: 107-75-5
📘 FEMA: Not listed
⚖️ MW: 172.26 g/mol
📝 Odor Type: Floral (Muguet)
📈 Odor Strength: Medium
👃🏼 Odor Profile: Sweet, floral, light, and clean with muguet character; tenacity increases in blends; evolves into a more powerful floral tone
👅 Flavor Profile: Sweet and floral with a bitter aftertaste above 20 ppm
⚗️ Uses: Core material in muguet, peony, sweet pea, narcissus; effective at 1–30% depending on application; also used in soaps, lipsticks, and berry flavor profiles
🧴 Appearance: Clear, colorless to pale liquid; susceptible to oxidation and polymerization on blotters
What is Hydroxycitronellal?
Hydroxycitronellal (7-hydroxy-3,7-dimethyloctanal) is a synthetic medium-chain aldehyde derived from citronellal. First synthesized by Knoll & Co. in the early 1900s and later refined by Bedoukian in 1967, it has since become a benchmark floralizer in perfumery. It belongs to the aldehyde class but contains both a hydroxyl and an aldehydic group, contributing to its unique olfactory and functional behavior.
Its primary odor profile is floral, closely resembling muguet (lily of the valley), with a mild, clean freshness. Over time and in proper blends, its olfactory strength increases markedly, making it a valued tenacious element in floral bases.
Olfactory Profile & Perfumery Applications
Hydroxycitronellal is used in both fine and functional perfumery. Its role as a floralizer makes it nearly indispensable in:
Muguet and peony bases
White florals: sweet pea, narcissus, and linden blossom
Soaps and detergents: strong performance in “rough” grades
Lipstick fragrances: adds roundness and floral sweetness
Rose undertones in modern compositions
Fruity flavors: adds depth to strawberry, cherry, and violet complexes
Its contribution to diffusion and body is highly valued in aldehydic florals and sweet, fruity bases. Pure grades offer elegance and diffusion, while industrial grades are favored in high-resistance formulations like soap.
Historical Background & Discovery
First marketed between 1905 and 1908 by Knoll & Co. (now part of BASF), hydroxycitronellal entered public use in the 1920s. Its prominent role in post-war perfumery began with the launch of L’Air du Temps and continued with Dior, Balenciaga, and Balmain. Despite being over a century old, its use continues to grow globally.
The first commercial large-scale usage dates to the 1940s. For over 40 years, hydroxycitronellal had no true substitute in muguet-type perfumes. Even after the development of modern alternatives, demand has remained consistent.
Production and Synthesis
Hydroxycitronellal is synthesized via hydration of citronellal or via oxazolidine intermediates. One typical route involves:
Reaction of citronellal with diethanolamine → oxazolidine
Dissolution in 98% sulfuric acid → sulfate ester intermediate
Hydrolysis in aqueous medium → Hydroxycitronellal
The compound’s structure contributes to its surface activity, enabling it to reduce viscosity or break emulsions—properties relevant for functional product performance.

Regulatory & Safety Overview
IFRA Status: Subject to concentration limits in consumer products
EU Cosmetics Regulation (1223/2009): Listed among the 26 fragrance allergens; must be declared above threshold
RIFM Findings: Classified as a weak sensitizer; 98.3% of test subjects reported no reaction in patch tests
Toxicology: Low systemic toxicity; recommended use below defined IFRA thresholds
Storage Considerations: Sensitive to oxidation and polymerization; blotter testing subject to air degradation
Additional Notes on Performance
Tenacity: High in proper blends, despite medium vapor pressure
Compatibility: Performs better when blended with low-boiling modifiers
Evaluation Tip: Can be assessed in lukewarm water to expose impurities
Polarity Effects: Competes for micelle surface space, influencing viscosity and emulsion structure
Flavor Usage: Discrete presence (0.3–15 ppm) improves richness in citrus, berry, and violet flavor compositions
Sources
Scentspiracy-provided source
Perfume and Flavor Chemicals (S. Arctander, 1969)
C. Sell, Chemistry and the Sense of Smell
National Center for Biotechnology Information – PubChem
RIFM Safety Assessment Reports
IFRA Standards Documentation
De Monchy Aromatics Technical Papers