Image 1 of 2
Image 1 of 2

 Image 2 of 2
Image 2 of 2



Geraniol Fine 98%
Premium Synthetic Ingredient for Perfumery
Geraniol Fine 98% is a high-purity synthetic floral alcohol with a characteristic sweet, warm, rose-like scent, often used to build peony, magnolia, sweet pea, and ylang-ylang accords. Found naturally in many essential oils including rose, geranium, and citronella, geraniol is a versatile floral backbone material in both fine fragrance and functional perfumery. Its balance of softness and persistence allows controlled use across diverse olfactory structures. Geraniol is also a precursor in citral synthesis for vitamin A production, making it valuable beyond scent formulation.
Premium Synthetic Ingredient for Perfumery
Geraniol Fine 98% is a high-purity synthetic floral alcohol with a characteristic sweet, warm, rose-like scent, often used to build peony, magnolia, sweet pea, and ylang-ylang accords. Found naturally in many essential oils including rose, geranium, and citronella, geraniol is a versatile floral backbone material in both fine fragrance and functional perfumery. Its balance of softness and persistence allows controlled use across diverse olfactory structures. Geraniol is also a precursor in citral synthesis for vitamin A production, making it valuable beyond scent formulation.
Premium Synthetic Ingredient for Perfumery
Geraniol Fine 98% is a high-purity synthetic floral alcohol with a characteristic sweet, warm, rose-like scent, often used to build peony, magnolia, sweet pea, and ylang-ylang accords. Found naturally in many essential oils including rose, geranium, and citronella, geraniol is a versatile floral backbone material in both fine fragrance and functional perfumery. Its balance of softness and persistence allows controlled use across diverse olfactory structures. Geraniol is also a precursor in citral synthesis for vitamin A production, making it valuable beyond scent formulation.
Synthetic Ingredient Overview
🏭 Manufacturer — Symrise
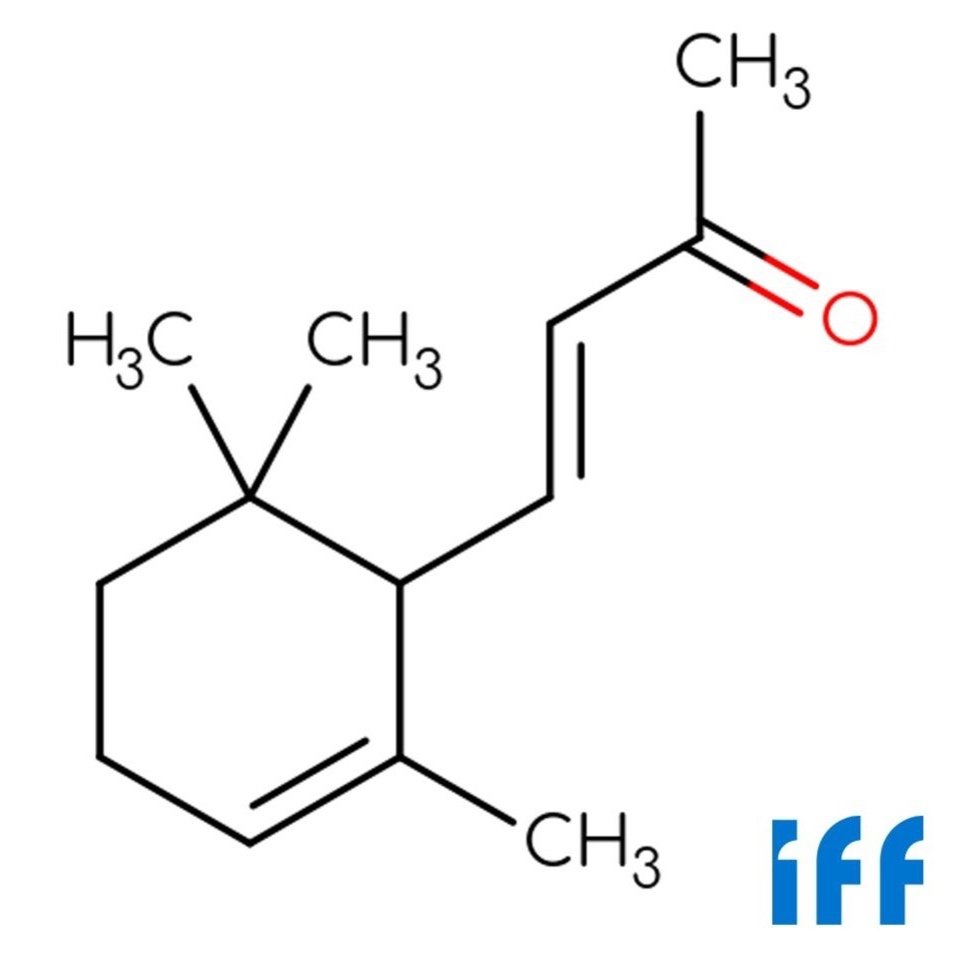
🔎 Chemical name — (2E)-3,7-Dimethylocta-2,6-dien-1-ol
🧪 Synonyms — Geraniol, Rhodinol, Lemonol (historical), Licarhodol
🧬 Chemical Formula — C₁₀H₁₈O
📂 CAS N° — 106-24-1
📘 FEMA — 2507
⚖️ MW — 154.25 g/mol
📝 Odor type — Floral / Rosy
📈 Odor Strength — Medium
👃🏼 Odor Profile — Mild, sweet, floral, rose-type with warm and slightly dry nuances. Varies by origin and method of production
⚗️ Uses — Major component in ylang-ylang bases and rose accords; also used in peony, sweet pea, frangipani, magnolia, neroli and fantasy florals
🧴 Appearance — Colorless to pale yellow liquid
💧 Solubility — Slightly soluble in water, miscible with alcohol and oils, soluble in propylene glycol
What is Geraniol?
Geraniol is a terpenoid alcohol classified among the “rose alcohols” alongside nerol, linalool, and citronellol. First isolated by Oscar Jacobsen in 1871 from Andropogon schoenanthus (geranium grass), it was historically known under several names including rhodinol, lemonol, and licarhodol, contributing to taxonomic confusion.
Chemically identified as C₁₀H₁₈O, geraniol is distinguished from its isomer nerol by its higher boiling point and lack of optical activity. It was conclusively identified in Turkish and German rose oil by Bertram and Gildemeister through calcium chloride complexation.
Geraniol occurs widely in nature and is a key constituent in palmarosa, rose, citronella, lemongrass, and ylang-ylangessential oils. Historically, it has been one of the defining components in natural rose oil analysis.
Olfactory Profile and Perfumery Applications
Geraniol delivers a soft, warm rose-floral scent, making it indispensable in:
Ylang-ylang reconstructions
Sweet pea, peony, magnolia, frangipani, neroli creations
Oriental florals and fantasy floral compositions
Base and middle note harmonization
Despite being known as a “rose alcohol”, Geraniol extends its role well beyond rose-centric perfumes, often serving as a sweetening, floralizing agent for full-bodied white florals and tropical blends.
Synergies & Comparisons
Geraniol blends synergistically with Linalool, Citronellol, and Nerol, enhancing floral warmth and persistence
Compared to Linalool, Geraniol is less volatile and more tenacious
Works particularly well in floral accords where citral, neral, or aldehydes are present, due to complementary chemical reactivity
Industrial and Technical Uses
Beyond perfumery, Geraniol serves as:
Precursor for citral, used in vitamin A synthesis
Flavoring agent in baked goods, beverages, and confections (GRAS status)
Antibacterial and insect-repellent additive in cosmetics and household care
Synthetic Routes
Isolation from citronella or palmarosa oil (via saponification and distillation)
From linalool: via isomerization using vanadate catalysts
From β-pinene: pyrolysis to myrcene → chlorides → geranyl acetate → saponification
These methods enable production of Geraniol >96–98% purity suitable for fine fragrance use.
Regulatory and Safety Overview
IFRA: Usage restricted in certain categories due to sensitization potential. Check category-specific limits
EU Allergen Listing: Listed among the 26 allergens (must be declared above threshold limits)
FEMA GRAS: Listed under FEMA No. 2507; approved for food use
ECHA: Registered; not classified as carcinogenic or mutagenic
Toxicology: May cause dermal irritation in sensitive individuals at high concentrations; oxidized forms are more allergenic
Stability: Oxidizes with air/light; storage in cool, sealed containers recommended
✅ Widely used under allergen labeling regulation; stable and effective when handled properly in fragrance formulation.