Citronellol 95
Synthetic Ingredient for Perfumery
Citronellol is a synthetic monoterpenoid alcohol widely used in perfumery for its clean, floral-rosy aroma with subtle leathery and waxy undertones. Known for its medium odor strength and versatility, it serves as a fundamental building block in rose accords, muguet types, and fresh-floral profiles. Available in both natural and synthetic forms, citronellol is valued for its ability to add volume, freshness, and tenacity to fragrance compositions. It also finds extensive use in flavors, soaps, and insect repellents.
Synthetic Ingredient for Perfumery
Citronellol is a synthetic monoterpenoid alcohol widely used in perfumery for its clean, floral-rosy aroma with subtle leathery and waxy undertones. Known for its medium odor strength and versatility, it serves as a fundamental building block in rose accords, muguet types, and fresh-floral profiles. Available in both natural and synthetic forms, citronellol is valued for its ability to add volume, freshness, and tenacity to fragrance compositions. It also finds extensive use in flavors, soaps, and insect repellents.
Synthetic Ingredient for Perfumery
Citronellol is a synthetic monoterpenoid alcohol widely used in perfumery for its clean, floral-rosy aroma with subtle leathery and waxy undertones. Known for its medium odor strength and versatility, it serves as a fundamental building block in rose accords, muguet types, and fresh-floral profiles. Available in both natural and synthetic forms, citronellol is valued for its ability to add volume, freshness, and tenacity to fragrance compositions. It also finds extensive use in flavors, soaps, and insect repellents.
Synthetic Ingredient Overview
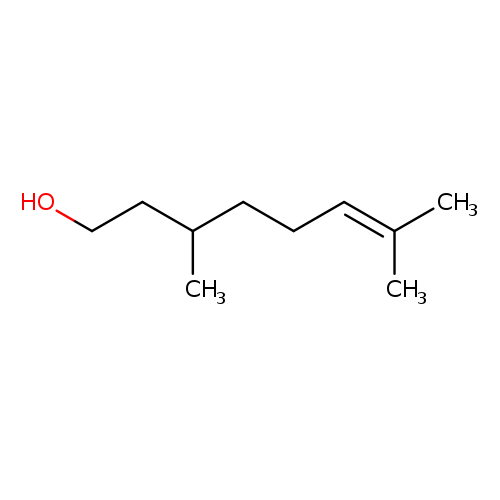
🔎 Chemical Name — 3,7-dimethyloct-6-en-1-ol
📂 CAS N° — 106-22-9
⚖️ Molecular Weight — 156.26 g/mol
📝 Odor Type — Floral (Rosy)
📈 Odor Strength — Medium
👃🏼 Odor Profile — Clean, rosy, floral, with leather-waxy undertones and citrus-green nuances; reminiscent of geranium and citronella
⚗️ Uses — Core material for rose and muguet accords, fresh floral perfumes, soaps, home care, low-cost compositions; flavor use in berry, citrus, and floral themes
🧴 Appearance — Clear, colorless to pale yellow liquid
What is Citronellol?
Citronellol is a monoterpenoid alcohol with chirality, present as (+)- and (−)-enantiomers in nature. The (+)-isomer is dominant in citronella oil, while the (−)-isomer is found in rose and geranium oils. Commercial Citronellol (95% purity or higher) often contains trace amounts of geraniol and other terpenoids.
In perfumery, Citronellol is essential for rosy and fresh-floral creations, acting as a backbone in compositions ranging from fine fragrances to functional products. It blends seamlessly with phenylethyl alcohol, geraniol, linalool, and aldehydes, offering a bright, dewy floral character.
Olfactory Profile and Applications in Perfumery
Citronellol is prized for its:
Rich rosy character, forming the base of most rose accords
Sweet-floral and citrus top facets, adding lift and diffusion
Leather-waxy and green terpene undertones, contributing complexity
Versatility across price ranges, from premium scents to mass-market soaps
Used in:
Rose, muguet, lily-of-the-valley, and peony bouquets
Citrus-floral and fruity-floral combinations
Home care and laundry fragrances
Traditional and modern perfumery bases
Usage concentrations vary:
Fine fragrance: 0.05% – 3%
Soap and detergent: 0.1% – 1.5%
Flavor: typically <10 ppm (e.g. raspberry, citrus, cherry, peach)
Industrial, Flavor, and Insecticidal Applications
Flavor Use — Citronellol is FEMA GRAS (#2309); used in berry, rose, raspberry, and citrus flavorings
Insect Repellency — Known for repelling mosquitoes; often complexed with β-cyclodextrin for formulation stability
Functional Products — Soaps, detergents, shampoos, and room sprays benefit from its rosy-clean note
Chemistry and Production
Synthesis pathways include:
Hydrogenation of geraniol or nerol
Reduction of citronellal (yielding high-purity citronellol)
From pinene, as part of synthetic routes to hydroxycitronellal
Structurally:
Acyclic monoterpenoid alcohol
Chirality affects odor character; industrial product is often racemic
Solubility:
Miscible with alcohol, propylene glycol, and oils
Very slightly soluble in water
Not soluble in glycerin
Safety and Regulatory Overview
IFRA Restrictions — Subject to concentration limits due to sensitization potential
EU Allergen Listing — Declaration required in cosmetics >0.001% (rinse-off) or >0.01% (leave-on)
FEMA GRAS — 2309
GHS Classification:
H315 – Causes skin irritation
H317 – May cause allergic skin reaction
H319 – Causes serious eye irritation
H401 – Toxic to aquatic life
Handling Precautions (P-statements):
P261, P264, P272, P273, P280, P302+P352, P305+P351+P338, P333+P313, P337+P313, P362, P501
✅ Controlled and informed use in fragrance compositions is recommended.
Sources
S. Arctander – Perfume and Flavor Materials of Natural Origin
Chirality & Odour Perception, J. Leffingwell, Ph.D.
Lawless, J. (1995). The Illustrated Encyclopedia of Essential Oils
National Center for Biotechnology Information (2020), PubChem CID 8842
Fulvio Ciccolo, Creative Base Design Notes
Formulation Stability Testing Notes – Scentspiracy Studio (2024)
Perfumery Raw Materials Handbook, Internal Training (2023)