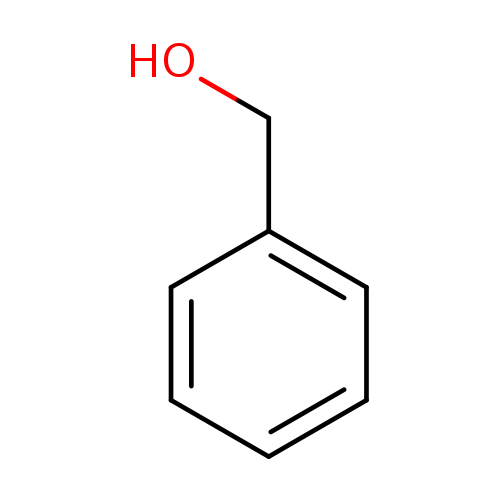
Benzyl Alcohol
Premium Synthetic Ingredient for Perfumery
Benzyl Alcohol is a synthetic aromatic alcohol widely employed as a functional perfumery solvent and floral blender. Known under the IUPAC name phenylmethanol, it is characterized by a faint, slightly sweet, almond-like scent. While its odor impact is subtle, Benzyl Alcohol serves a critical modulatory role in floral compositions such as Jasmine, Lilac, and Gardenia, smoothing transitions and enhancing note cohesion. It is also valued for its solubilizing capacity and compatibility with natural oils, absolutes, and other solvents.
Though not indispensable in every formulation, it remains a fundamental tool for perfumers seeking precision in complex floral arrangements.
Premium Synthetic Ingredient for Perfumery
Benzyl Alcohol is a synthetic aromatic alcohol widely employed as a functional perfumery solvent and floral blender. Known under the IUPAC name phenylmethanol, it is characterized by a faint, slightly sweet, almond-like scent. While its odor impact is subtle, Benzyl Alcohol serves a critical modulatory role in floral compositions such as Jasmine, Lilac, and Gardenia, smoothing transitions and enhancing note cohesion. It is also valued for its solubilizing capacity and compatibility with natural oils, absolutes, and other solvents.
Though not indispensable in every formulation, it remains a fundamental tool for perfumers seeking precision in complex floral arrangements.
Premium Synthetic Ingredient for Perfumery
Benzyl Alcohol is a synthetic aromatic alcohol widely employed as a functional perfumery solvent and floral blender. Known under the IUPAC name phenylmethanol, it is characterized by a faint, slightly sweet, almond-like scent. While its odor impact is subtle, Benzyl Alcohol serves a critical modulatory role in floral compositions such as Jasmine, Lilac, and Gardenia, smoothing transitions and enhancing note cohesion. It is also valued for its solubilizing capacity and compatibility with natural oils, absolutes, and other solvents.
Though not indispensable in every formulation, it remains a fundamental tool for perfumers seeking precision in complex floral arrangements.
Technical Ingredient Overview
🔎 Chemical Name — Benzyl Alcohol
🧪 Synonyms — Phenylmethanol, α-Hydroxytoluene, Benzenemethanol
🧬 Chemical Formula — C₆H₅CH₂OH
📂 CAS — 100-51-6
📘 FEMA — 2137
⚖️ MW — 108.14 g/mol
📝 Odor Type — Aromatic-Alcoholic
📈 Odor Strength — Moderate to Medium
👃🏼 Odor Profile — Mild, sweet-balsamic, faintly floral, with almond and faintly spicy nuances
⚗️ Uses — Solvent, fixative, preservative, intermediate, aromatic alcohol in perfumery
🧴 Appearance — Clear, colorless to slightly yellow, oily liquid
What is Benzyl Alcohol?
Benzyl Alcohol (CAS 100-51-6) is an aromatic alcohol composed of a phenyl group attached to a hydroxymethyl group. Naturally present in many essential oils—most notably in jasmine, hyacinth, and ylang-ylang—it also occurs in fruits such as apricots and cranberries. In the fragrance and flavor industries, it is both synthetically produced and derived from natural sources. Benzyl Alcohol is prized for its dual functionality as a mild-smelling aromatic agent and as a high-boiling-point solvent in perfume compositions.
Historical Background
Benzyl Alcohol has long held a dual role in both natural extraction and synthetic chemistry, with a documented history that predates the modern perfume industry. It was first isolated from benzyl compounds in the early 19th century, closely following developments in the structural elucidation of aromatic alcohols. According to historical records, its synthesis is attributed to Friedrich Wöhler and Justus von Liebig, who explored the oxidation and reduction behavior of benzyl derivatives during their pioneering studies in organic chemistry (Liebig & Wöhler, 1832).
The commercial synthesis of Benzyl Alcohol developed rapidly in the late 19th and early 20th centuries, using processes such as the hydrolysis of benzyl chloride—a method still relevant today. This made it economically viable and opened its use to various industrial domains, including pharmaceuticals, dyes, and later, perfumery.
In fragrance history, Benzyl Alcohol gained prominence not only for its own soft floral-balsamic note, but as a precursor to esters such as benzyl acetate and benzyl benzoate—materials that define jasmine, gardenia, and tuberose accords. The importance of Benzyl Alcohol surged in the early 20th century, especially after the creation of synthetic jasmine bases which relied on combinations of benzyl acetate, methyl anthranilate, and benzyl alcohol to reconstruct the complex natural aroma of jasmine absolute at lower cost.
Renowned perfumer and chemist Ernest Beaux is documented to have worked with benzyl-derived materials in the early Chanel No. 5 formula, capitalizing on their ability to soften aldehydes and enrich floral heart notes. Benzyl Alcohol’s presence in natural essential oils such as ylang-ylang, jasmine, and styrax also reinforced its status as a naturally occurring molecule that bridges both the botanical and synthetic worlds (Arctander, 1960).
By mid-20th century, the molecule was firmly established in perfumery as both an odorant and technical agent—used for its fixative behavior and solvent capabilities, especially in soap perfumes, colognes, and floral compositions. Its mildness, combined with relatively low cost and multifunctionality, ensured its continued use across fragrance genres.
Olfactory Profile
Scent Family: Aromatic-Alcoholic
Main Descriptors: Mild, sweet, slightly balsamic, floral, faintly almondic
Tenacity: Low to moderate
Volatility: Medium (b.p. 205–207 °C)
Fixative Role: Moderate, supports base notes
In perfumery, Benzyl Alcohol is often used to round off sharp floral notes or to add subtle sweetness to a composition. It harmonizes well with white floral ingredients like benzyl acetate, phenyl ethyl alcohol, and methyl anthranilate, making it a valuable building block in jasmine and lilac reconstructions. Though relatively weak in projection, its utility as a solvent and fixative enhances the tenacity of volatile top and mid-notes.
Notable uses:
Floral compositions (especially white flowers)
Soap perfumes and functional fragrance bases
Almond-type accords (in synergy with benzaldehyde)
Industrial & Technical Uses
Beyond its olfactory properties, Benzyl Alcohol is an extensively used solvent in a variety of technical applications. These include:
Solvent for inks, paints, and epoxy resins
Preservative in pharmaceutical and cosmetic products
Intermediate in the synthesis of esters (e.g., benzyl acetate)
Anesthetic agent in injectable formulations
Plasticizer in cellulose acetate materials
Its relatively low toxicity compared to other aromatic solvents makes it suitable for topical and parenteral formulations in pharma and personal care industries.
Regulatory & Safety Overview
IFRA Status: Benzyl Alcohol is listed by the IFRA Standards (up to Amendment 51) as a potential allergen. It is allowed within concentration limits depending on the application category (e.g., <1% in leave-on cosmetics).
EU Cosmetic Regulation (EC) No 1223/2009: Benzyl Alcohol is one of the 26 declarable fragrance allergens, and must be listed on product labels if concentration exceeds 0.001% in leave-on or 0.01% in rinse-off products.
GHS Classification:
Acute toxicity (oral, dermal, inhalation): Category 4
Eye irritation: Category 2A
Signal word: Warning
Toxicology:
Benzyl Alcohol is metabolized primarily to benzoic acid and excreted as hippuric acid. In high concentrations or prolonged use, it may cause skin sensitization or contact dermatitis.
REACH Compliance: Registered under EU REACH regulation. No current restrictions on its use in perfumery or cosmetics within the specified limits.
FEMA GRAS: Listed as GRAS (Generally Recognized As Safe) for flavor use by FEMA (No. 2137).
Additional Information
Natural Occurrence:
Occurs naturally in jasmine (Jasminum grandiflorum), ylang-ylang (Cananga odorata), hyacinth, benzoin resinoid, and cinnamon leaf oil.
Key Synthesis Routes:
Hydrolysis of benzyl chloride
Reduction of benzaldehyde
Cannizzaro reaction (with benzaldehyde under basic conditions)
Related Ingredients for Internal Linking:
Benzyl Acetate — a fruity-floral ester from benzyl alcohol
Phenyl Ethyl Alcohol — another floral alcohol with rosey nuances
Methyl Anthranilate — nitrogenous floral molecule frequently co-used in white floral accords
References
Arctander, S. (1960). Perfume and Flavor Chemicals (Aroma Chemicals). Montclair, NJ: Author.
Sell, C. S. (2019). Chemistry and the Sense of Smell. Wiley.
Sell, C. S. (2006). The Chemistry of Fragrances. Royal Society of Chemistry.
European Commission. (2009). Regulation (EC) No 1223/2009 on Cosmetic Products. Official Journal of the European Union.
PubChem. (2024). Benzyl Alcohol. https://pubchem.ncbi.nlm.nih.gov/compound/244
FEMA. (2020). Flavor Ingredient Library - Benzyl Alcohol (2137). www.femaflavor.org
Kraft, P. (2013). Aromachemicals in Perfumery: Synthesis and History. In: Chemistry & Biodiversity.
Liebig, J. & Wöhler, F. (1832). Untersuchungen über das Radikal der Benzoesäure. Annalen der Pharmacie.
Gildemeister, E., & Hoffmann, F. (1913). The Volatile Oils. Macmillan.
IFRA (2023). IFRA Standards 51st Amendment.