Image 1 of 3
Image 1 of 3

 Image 2 of 3
Image 2 of 3

 Image 3 of 3
Image 3 of 3




Alpha Pinene
Premium Synthetic Ingredient for Perfumery
Alpha-Pinene (CAS 80-56-8) is a naturally derived monoterpene hydrocarbon widely used in perfumery for its bright, resinous pine aroma. Found in the essential oils of conifers such as Pinus sylvestris and Pinus pinaster, it serves as a volatile top note and green modifier across woody, fougère, citrus, and aromatic fragrance types.
Its combination of high diffusivity, natural-smelling freshness, and strong terpenic identity makes it indispensable in both artistic and functional formulations.
Premium Synthetic Ingredient for Perfumery
Alpha-Pinene (CAS 80-56-8) is a naturally derived monoterpene hydrocarbon widely used in perfumery for its bright, resinous pine aroma. Found in the essential oils of conifers such as Pinus sylvestris and Pinus pinaster, it serves as a volatile top note and green modifier across woody, fougère, citrus, and aromatic fragrance types.
Its combination of high diffusivity, natural-smelling freshness, and strong terpenic identity makes it indispensable in both artistic and functional formulations.
Premium Synthetic Ingredient for Perfumery
Alpha-Pinene (CAS 80-56-8) is a naturally derived monoterpene hydrocarbon widely used in perfumery for its bright, resinous pine aroma. Found in the essential oils of conifers such as Pinus sylvestris and Pinus pinaster, it serves as a volatile top note and green modifier across woody, fougère, citrus, and aromatic fragrance types.
Its combination of high diffusivity, natural-smelling freshness, and strong terpenic identity makes it indispensable in both artistic and functional formulations.
Technical Ingredient Overview
🏭 Manufacturer — Firmenich
🔎 Chemical Name — Alpha-Pinene
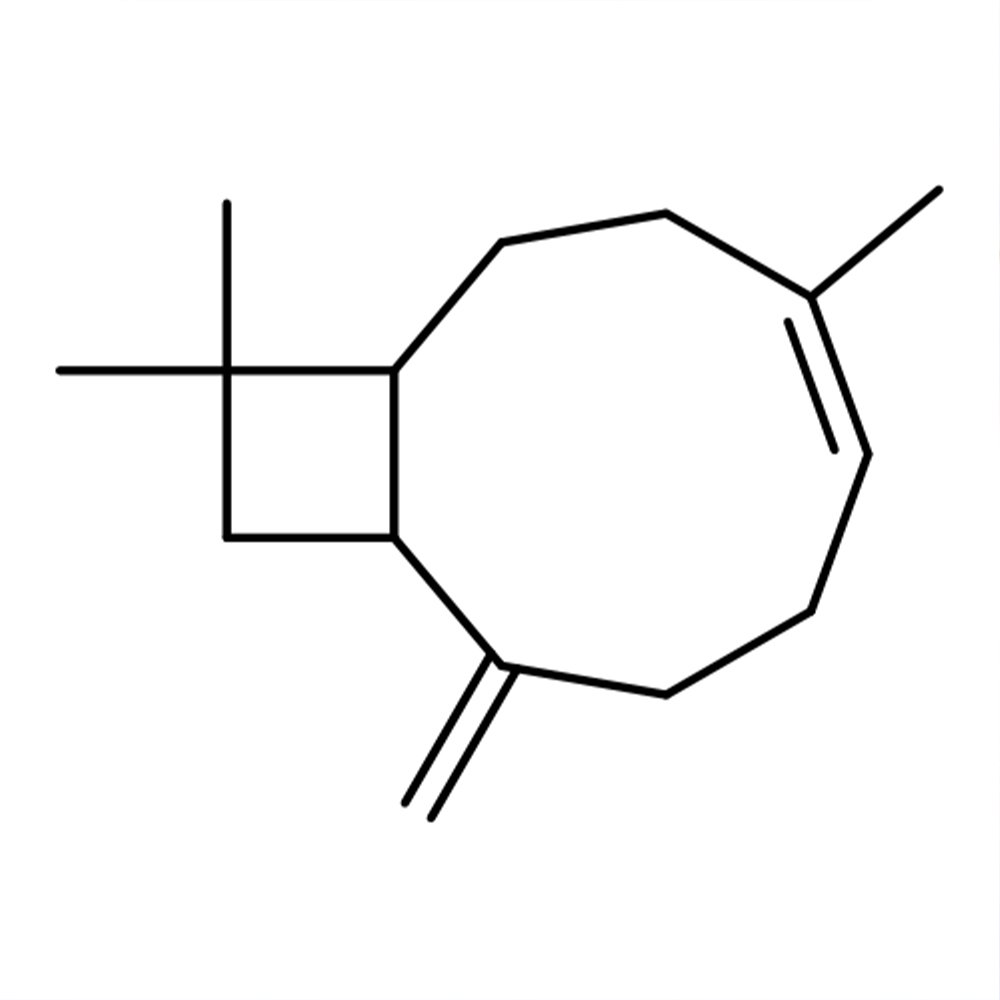
🧪 Synonyms — α-Pinene, (1S)-2,6,6-trimethylbicyclo[3.1.1]hept-2-ene
🧬 Chemical Formula — C₁₀H₁₆
📂 CAS — 80-56-8
📘 FEMA — 2902
⚖️ MW — 136.24 g/mol
📝 Odor Type — Woody, balsamic
📈 Odor Strength — Strong
👃🏼 Odor Profile — Fresh pine, resinous, turpentine-like, slightly herbal
⚗️ Uses — Fragrance in fine perfumery, household cleaners, flavoring, solvent, biosynthetic precursor
🧴 Appearance — Colorless to pale yellow liquid
What is Alpha-Pinene?
Alpha-Pinene is a bicyclic monoterpene hydrocarbon widely found in nature and among the most abundant terpenes emitted by forest vegetation. It occurs predominantly in coniferous trees (notably in Pinus species), where it is a major constituent of turpentine oil. It exists as two enantiomers: (+)-α-pinene (typical of European pines) and (–)-α-pinene (common in North American pines). Its volatility and strong piney odor make it highly relevant in perfumery, flavoring, and also in industrial chemistry as a feedstock for other terpene derivatives (Sell, 2019; Arctander, 1960).
Historical Background
The distillation of turpentine oil from pine resin, and the subsequent isolation of α-pinene, dates back to the 19th century with the rise of industrial chemistry in Europe. However, its presence and use precede this by centuries through the folk use of pine resins and oils in traditional medicine and incense. Alpha-Pinene was among the first monoterpenes characterized chemically, with foundational structural elucidation work done in the late 1800s and early 1900s (Simonsen & Ross, 1932). Its role in fragrance chemistry was solidified in the early 20th century due to its contribution to pine accords and later as a synthetic intermediate in terpene chemistry.
Olfactory Profile
Scent Family: Terpenic / Woody / Green
Main Descriptors: Pine-like, resinous, camphoraceous, slightly herbal
Intensity: High; diffusive and sharp
Volatility: High (top note)
Fixative Role: Minimal due to high volatility
In fine perfumery, α-pinene imparts a fresh, forest-like aroma. It forms the backbone of pine and coniferous accords and is often used to bring brightness to fougère, chypre, and fresh woody compositions. It is typically paired with other terpenes, aldehydes, and balsamic materials. Despite its reactivity, it contributes a fresh, invigorating lift when dosed judiciously. Notable commercial use includes fresh pine-based room sprays and deodorants.
Performance in Formula:
Alpha-Pinene displays excellent top note diffusion but poor tenacity. It blends well with limonene, eucalyptol, camphene, borneol, and other monoterpenes. It oxidizes relatively quickly upon exposure to air, which must be accounted for in formulation to avoid degradation or allergenic by-products such as pinocarvone and pinene oxide (Sköld et al., 2002).
Industrial & Technical Uses
Beyond perfumery, α-pinene serves as a valuable intermediate in the synthesis of camphor, synthetic sandalwood analogs, and other terpenoids. It is used as:
A flavoring agent (e.g., in beverages and confectionery)
A solvent or carrier in household and industrial cleaning products
A starting material for the production of synthetic fragrances and pharmaceuticals
A precursor in the synthesis of sobrerol, verbenone, and other oxygenated terpenes
Its availability from renewable pine resin makes it attractive in green chemistry and biosynthetic applications (Bicas et al., 2011).
Regulatory & Safety Overview
IFRA Status: As of IFRA Amendment 51, α-pinene is not restricted, but its oxidation products (e.g., pinene oxide) are classified as sensitizers. Formulators should avoid use of oxidized material.
GHS Classification:
Flammable liquid (Category 3)
Skin Irritant (Category 2)
Skin Sensitizer (Category 1)
Aquatic Toxicity (Chronic Category 1)
EU Cosmetics Regulation (EC 1223/2009): Permitted; labeling may be required if used in products with exposure limits above thresholds for oxidized derivatives.
REACH: Registered under REACH with safety data available
FEMA GRAS: Listed as GRAS (Generally Recognized As Safe) for use in food up to specified levels
Proper storage in airtight containers, under inert gas if possible, is essential to prevent oxidative degradation and potential allergen formation.
References
Arctander, S. (1960). Perfume and Flavor Chemicals (Aroma Chemicals). Self-published.
Bicas, J. L., Silva, J. C., & Pastore, G. M. (2011). Terpene biotransformations for the production of fine chemicals. Biotechnology Advances, 29(1), 1–14. https://doi.org/10.1016/j.biotechadv.2010.08.008
Sell, C. S. (2019). The Chemistry of Fragrances: From Perfumer to Consumer (3rd ed.). Royal Society of Chemistry.
Simonsen, J. L., & Ross, W. C. J. (1932). The Terpenes. Cambridge University Press.
Sköld, M., Börje, A., Harambasic, E., & Karlberg, A. T. (2002). Contact allergens formed on air exposure of linalool. Contact Dermatitis, 46(6), 306–312. https://doi.org/10.1034/j.1600-0536.2002.460502.x