 Image 1 of 3
Image 1 of 3

 Image 2 of 3
Image 2 of 3

 Image 3 of 3
Image 3 of 3




1-OCTENE-3-OL
Premium Synthetic Ingredient for Perfumery
1-Octen-3-ol is a synthetic unsaturated alcohol derived from fatty acid oxidation, classified among C8 alcohols. It emits a distinctive mushroom-like, earthy-green scent with metallic and slightly fatty undertones. This compound is not a fixative but functions as a top-to-heart note modifier in perfumery, enhancing realism in green, naturalistic, and damp soil-inspired accords. Due to its high diffusion and low odor threshold, it is used sparingly for olfactory contrast and natural depth.
Premium Synthetic Ingredient for Perfumery
1-Octen-3-ol is a synthetic unsaturated alcohol derived from fatty acid oxidation, classified among C8 alcohols. It emits a distinctive mushroom-like, earthy-green scent with metallic and slightly fatty undertones. This compound is not a fixative but functions as a top-to-heart note modifier in perfumery, enhancing realism in green, naturalistic, and damp soil-inspired accords. Due to its high diffusion and low odor threshold, it is used sparingly for olfactory contrast and natural depth.
Premium Synthetic Ingredient for Perfumery
1-Octen-3-ol is a synthetic unsaturated alcohol derived from fatty acid oxidation, classified among C8 alcohols. It emits a distinctive mushroom-like, earthy-green scent with metallic and slightly fatty undertones. This compound is not a fixative but functions as a top-to-heart note modifier in perfumery, enhancing realism in green, naturalistic, and damp soil-inspired accords. Due to its high diffusion and low odor threshold, it is used sparingly for olfactory contrast and natural depth.
Technical Ingredient Overview
🏭 Manufacturer — (not specified)
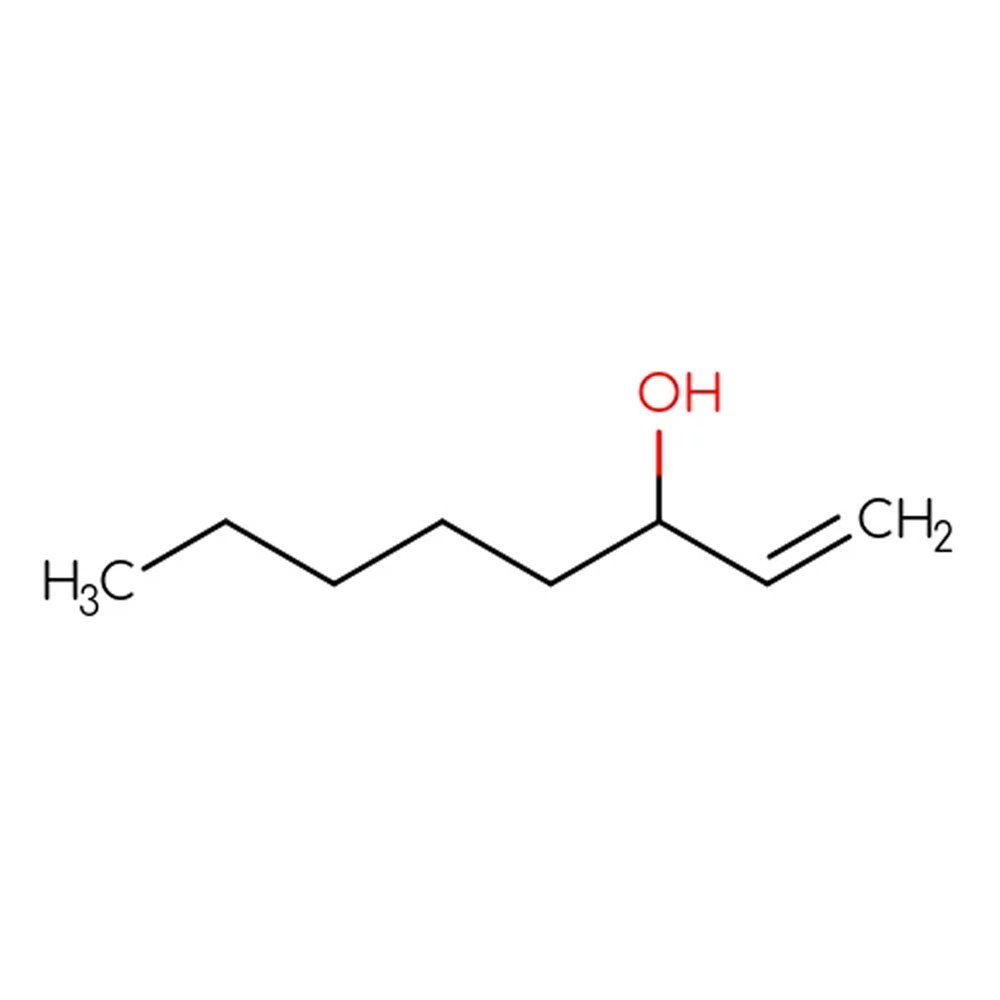
🔎 Chemical Name — 1-Octen-3-ol
🧪 Synonyms — Oct-1-en-3-ol, Mushroom alcohol, Octenol, Amyl Vinyl Carbinol
🧬 Chemical Formula — C₈H₁₆O
📂 CAS — 3391-86-4
📘 FEMA — 2805
⚖️ MW — 128.21 g/mol
📝 Odor Type — Green, Mushroom, Earthy
📈 Odor Strength — High
👃🏼 Odor Profile — Fresh cut mushroom, earthy, green, metallic, oily, slightly fatty
⚗️ Uses — Flavoring agent, perfumery raw material (green and natural effects), insect attractant/repellent
🧴 Appearance — Colorless to pale yellow liquid
What is 1-Octen-3-ol?
1-Octen-3-ol is an unsaturated alcohol derived from fatty acid oxidation and belongs to the class of C8 alcohols, known for their powerful green and earthy aromas. Commonly referred to as “mushroom alcohol”, this compound is a major volatile organic compound found in various fungi, especially edible mushrooms like Agaricus bisporus. It plays a critical role in flavor chemistry and perfume formulation due to its natural occurrence and strong impact at low concentrations.
Historical Background
First isolated in the mid-20th century during studies of fungal volatiles, 1-Octen-3-ol was soon recognized as a key contributor to mushroom aroma. It is biosynthesized through the enzymatic oxidation of linoleic acid, especially during fungal growth or cell disruption (Wurzenberger & Grosch, 1984). Its role in perfumery expanded with the rise of green and naturalistic notes in the 1970s–80s, paralleling developments in aroma chemical synthesis and headspace technology.
Olfactory Profile
Scent Family: Green, Earthy, Naturalistic
Main Descriptors: Mushroom, earthy, metallic-green, wet soil, slightly animalic
Intensity & Tenacity:
Very intense with a detection threshold ~0.005 ppm
Moderate tenacity, used in top-to-heart registersFixative Role: Not a fixative, but enhances realism in green/vegetal accords.
Applications in Fine Fragrance
1-Octen-3-ol is ideal for evoking humid forests, wet soil, or mushroomy undergrowth and features in:
Chypre, fougère, and natural green florals
Hyperrealistic niche creations
Mossy, damp, and forest-inspired accords
Earthy violet-leaf or vegetal accords
Blending with geosmin, methyl jasmonate, and vetiveryl acetate for natural effect
Ozonic or rain-type themes mimicking petrichor and humidity
Fougère and chypre fragrances for added depth and earthiness
Green florals, especially violet leaf, narcissus, and galbanum
Used sparingly, it adds realism and complexity to green compositions. Pairs especially well with materials like cis-3-hexenol, isobutyl quinoline, and vetiver derivatives.
Performance in Formula
Blend behavior: Highly diffusive; overuse easily dominates
Diffusion: Excellent projection and atmospheric presence
Compatibility: Ideal with other green materials, earthy woods, ozonic notes, violet leaf, and truffle-like molecules.
Industrial & Technical Uses
Flavor industry: FEMA 2805, for mushroom/truffle flavor notes
Insect research: Strong mosquito attractant; used in traps and vector control
Air quality testing: Biomarker for fungal contamination in indoor environments
Regulatory & Safety Overview
IFRA Status: Not restricted (IFRA 51, 2023)
GHS Classification:
H302: Harmful if swallowed
H315: Causes skin irritation
H317: May cause allergic skin reaction
EU Cosmetics Regulation: Permitted with labeling requirements above thresholds
REACH: Registered and compliant (see ECHA dossier)
FEMA: 2805 — GRAS for flavoring purposes
Allergen Risk: Moderate; patch testing advised in leave-on products
References
Arctander, S. (1960). Perfume and Flavor Chemicals (Aroma Chemicals). Montclair: Author.
Wurzenberger, M., & Grosch, W. (1984). Enzymic formation of 1-octen-3-one and 1-octen-3-ol in mushroom (Agaricus bisporus). Journal of Agricultural and Food Chemistry, 32(2), 283–286. https://doi.org/10.1021/jf00122a037
Zawirska-Wojtasiak, R., et al. (2009). Volatile mushroom flavor compounds and their biosynthesis. Acta Scientiarum Polonorum Technologia Alimentaria, 8(3), 73–81.
Syed, Z., & Leal, W. S. (2009). Acute olfactory response of Culex quinquefasciatus mosquitoes to a human-derived attractant. PNAS, 106(44), 18803–18808. https://doi.org/10.1073/pnas.0906932106
European Chemicals Agency (ECHA). (2024). Substance information: 1-Octen-3-ol. https://echa.europa.eu
Historical Context
1-Octen-3-ol, often referred to as “mushroom alcohol,” holds a unique and influential position in modern olfactory design. Unlike the dominant aldehydic florals and bright citrus notes that characterized much of 20th-century perfumery, this molecule brought with it a distinctly raw, earthy, and unapologetically natural scent profile. Its olfactory character—evocative of damp forest floors, fresh mushrooms, and crushed greenery—invited perfumers to engage with the concept of realism in a way that had previously been considered unorthodox or undesirable.
The adoption of 1-Octen-3-ol in perfumery coincided with a broader aesthetic and cultural shift. From the 1960s through the 1980s, there was a growing appetite for compositions that reflected nature more authentically. The emergence of green notes—galbanum, violet leaf, hexenals—challenged the dominance of polished floral and powdery profiles. These new accords introduced sharpness, bitterness, and verdancy into perfumery, paralleling rising cultural trends centered on environmentalism, natural beauty, and organic expression (Morris, 1981).
Within this context, 1-Octen-3-ol provided something that few materials could: a convincing representation of life beyond the flower—the soil, the fungus, the leaf in decay. Arctander (1969) recognized this early, describing it as “very powerful, sweet-earthy, almost buttery and fungus-like,” with facets of hay and herbaceousness. It was quickly embraced not as a central note, but as a modifier—a trace component that could transform a formula with remarkable effect. In fougère or lavender compositions, it added forest-floor realism. In green florals, it imparted shadow and depth. Even in delicate fragrances, a fractional percentage could add natural complexity (Wright, 2012).
Perfumers in this era began to embrace materials that conveyed imperfect, living nature, and 1-Octen-3-ol was emblematic of that movement. Its popularity paralleled the rise of niche and conceptual perfumery, where emotional landscapes—like rain, moss, and wilderness—required new olfactory tools. It was no longer about making nature smell better, but about making fragrance more truthful. As John Wright notes, 1-Octen-3-ol may not be “conventionally beautiful,” but it provides an irreplaceable earthy realism that few other molecules can match (Wright, 2012).
In essence, 1-Octen-3-ol helped define a moment in perfumery when rawness, naturality, and realism were not only accepted—they were celebrated.
Discovery and First Synthesis
The story of 1-Octen-3-ol begins not in a laboratory, but in the forest. In 1938, Japanese chemist S. Murahashi was the first to isolate and describe this molecule from the prized matsutake mushroom (Tricholoma matsutake), which he appropriately referred to as “matsutake alcohol” (Murahashi, 1938). This early isolation marked a turning point in the scientific understanding of the volatile compounds responsible for mushroom aroma.
Subsequent studies revealed that 1-Octen-3-ol is a product of enzymatic oxidation of linoleic acid, a fatty acid abundant in fungal tissue. When mushrooms are sliced or damaged, lipoxygenase enzymes catalyze the degradation of their membrane lipids, releasing volatile compounds—chief among them 1-Octen-3-ol (Wood et al., 2001). It was soon found not only in fungi, but in plants, essential oils (like pennyroyal and lavender), and even animal emissions, positioning it as a molecule of ecological significance.
Indeed, the molecule plays an important semiochemical role in nature. It has been shown to attract blood-feeding insects such as mosquitoes and tsetse flies, due to its presence in mammalian sweat and breath (Vale & Hall, 1985). It is also emitted by decaying vegetation and fungi as a signaling molecule in ecological networks. Some fungi may use it defensively to deter predators, as demonstrated in studies where it repelled banana slugs from mushroom caps (Wood et al., 2001). These findings have led to its use in mosquito bait formulations and insect behavior studies.
From a chemical perspective, once its structure was known, synthetic access to 1-Octen-3-ol became straightforward. The first laboratory syntheses employed a Grignard reaction, in which a vinyl magnesium halide was added to hexanal or acrolein, yielding racemic 1-octen-3-ol (Arctander, 1969). This approach offered high yields and was commercially viable. Over time, industrial production adopted more scalable techniques, including hydroformylation and selective oxidation of octenes. More recently, enantioselective methods have emerged to favor the (R)-enantiomer, which possesses the strongest mushroom character (Kraft, 2005).
By the late 1960s, synthetic 1-octen-3-ol was already being used to recreate or extend essential oils such as peppermint and lavender. Arctander (1969) notes its inclusion in the formulation of reconstituted oils, where a trace amount could dramatically increase fidelity. From this utilitarian function, it grew into a beloved material for its creative impact—a testament to how a modest, earthy alcohol from mushrooms helped rewrite the rules of olfactory storytelling.
References
Arctander, S. (1969). Perfume and Flavor Chemicals (Aroma Chemicals). Montclair, NJ: Author.
Burdock, G. A. (2010). Fenaroli’s Handbook of Flavor Ingredients (6th ed.). Boca Raton, FL: CRC Press.
ECHA. (2024). 1-Octen-3-ol – Substance Information. European Chemicals Agency. Retrieved from https://echa.europa.eu
Kraft, P. (2005). Olfactory properties of optical isomers: Recent examples in perfumery. Chimia, 59(4), 248–256.
Morris, A. F. (1981). Perfumery notes: Green notes. Perfumer & Flavorist, 6(1), 1–4.
Murahashi, S. (1938). On the components of matsutake aroma. Scientific Papers of the Institute of Physical and Chemical Research (Japan), 34, 155.
Sell, C. S. (2019). The Chemistry of Fragrances (3rd ed.). Cambridge: Royal Society of Chemistry.
Vale, G. A., & Hall, D. R. (1985). The use of 1-octen-3-ol, acetone, and carbon dioxide to improve baits for tsetse flies. Bulletin of Entomological Research, 75(2), 219–231.
Wood, W. F., Archer, C. L., & Largent, D. L. (2001). 1-Octen-3-ol, a banana slug antifeedant from mushrooms. Biochemical Systematics and Ecology, 29(5), 531–533.
Wright, J. (2012, August 13). Flavor bites: Earthy, musty, mushroomlike aroma chemicals. Perfumer & Flavorist. Retrieved from https://www.perfumerflavorist.com