 Image 1 of 2
Image 1 of 2

 Image 2 of 2
Image 2 of 2



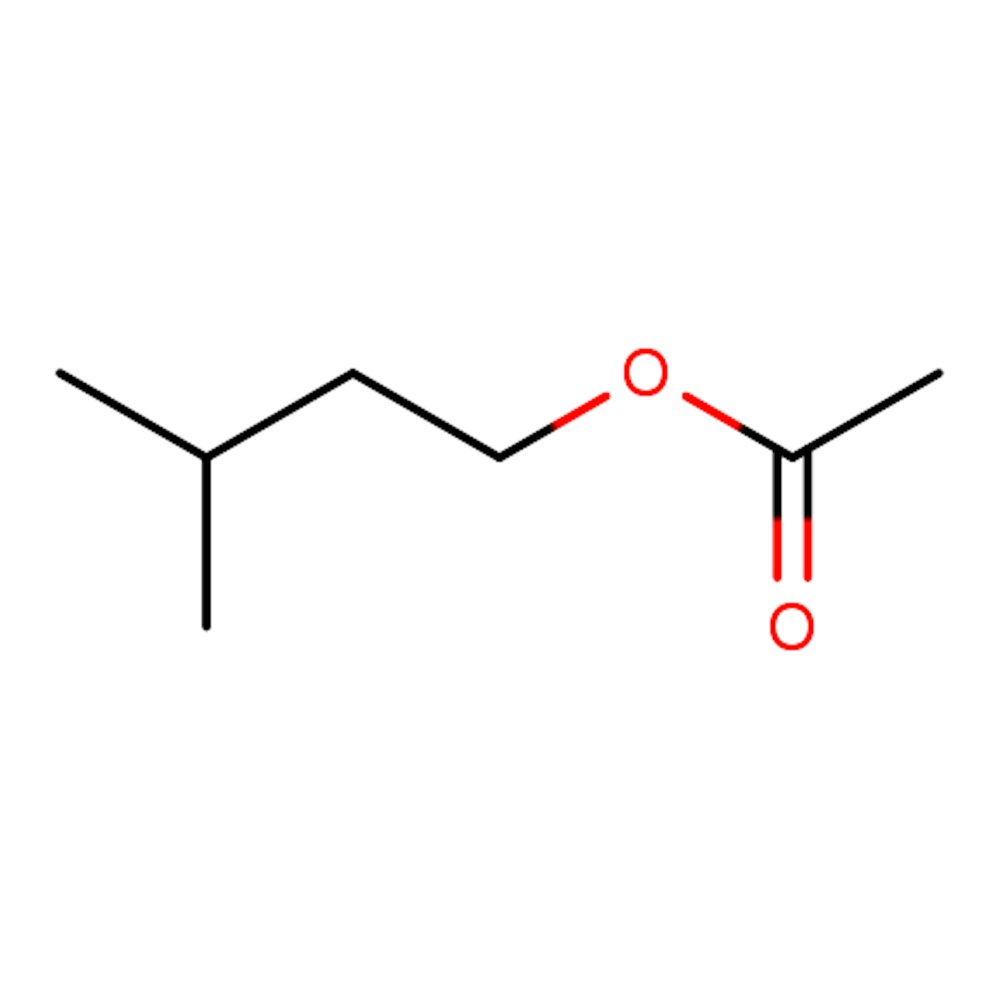
Isoamyl Acetate
Synthetic Ingredient For Perfumery
Isoamyl acetate, commonly known as isopentyl acetate or "Banana oil," is a colorless organic compound. It exhibits a potent aroma reminiscent of bananas and pears, formed from the esterification of isoamyl alcohol with acetic acid. This synthetic ingredient finds versatile applications in perfumery, imparting a fruity, ripe, and estery scent. Additionally, it serves as a solvent in various industrial contexts, including varnishes and aircraft dopes.
Synthetic Ingredient For Perfumery
Isoamyl acetate, commonly known as isopentyl acetate or "Banana oil," is a colorless organic compound. It exhibits a potent aroma reminiscent of bananas and pears, formed from the esterification of isoamyl alcohol with acetic acid. This synthetic ingredient finds versatile applications in perfumery, imparting a fruity, ripe, and estery scent. Additionally, it serves as a solvent in various industrial contexts, including varnishes and aircraft dopes.
Synthetic Ingredient For Perfumery
Isoamyl acetate, commonly known as isopentyl acetate or "Banana oil," is a colorless organic compound. It exhibits a potent aroma reminiscent of bananas and pears, formed from the esterification of isoamyl alcohol with acetic acid. This synthetic ingredient finds versatile applications in perfumery, imparting a fruity, ripe, and estery scent. Additionally, it serves as a solvent in various industrial contexts, including varnishes and aircraft dopes.
Synthetic Ingredient Overview
📂 CAS N° 123-92-2
🔎 Synonyms: Isopentyl acetate, 3-Methylbutyl acetate
⚗️Chemical Formula: C₇H₁₄O₂
📖 FEMA: 2055
📘 EINECS: 204-662-3
👁️ Appearance: Colorless, oily liquid
⚖️ MW — 130.18 g/mol
📝 Odor Type – Fruity.
📈 Odor Strength –High, recommended smelling at 1% sol. Stays 4hrs on paper at full concentration.
👃🏼 Odor Profile – sweet banana fruity ripe estery, solvent. Heady.
👅 Flavor Profile – banana, sweet, fruity, banana.
⚗️ Uses – Alcoholic Beverages, Apple, Apricot, Banana, Cherry, Chocolate, Cocoa, Dairy Products, Hard Fruits, Soft Fruits.
🚨 Recommended limit at 25% in the fragrance formula.
Historical Context
Isoamyl acetate, often referred to as the “banana ester,” is a classic example of a fruity-smelling ester used widely in both perfumery and flavor chemistry. Its strong association with banana and pear aromas has made it one of the most recognizable synthetic fruit notes since the early 20th century. The molecule gained notoriety not only for its sensory appeal but also for its role in training olfactory professionals due to its easily identifiable character (Sell, 2019).
Discovery and First Synthesis
Isoamyl acetate occurs naturally in bananas, apples, and other fruits, where it is biosynthesized via enzymatic esterification of isoamyl alcohol and acetic acid (Rowan, 2011). However, the first synthetic preparation dates back to the 19th century through classical esterification: heating isoamyl alcohol (a fusel alcohol from fermentation) with acetic acid in the presence of an acid catalyst (e.g., sulfuric acid). This method remains the basis for its industrial production today (Smith et al., 2006).
First Uses
The compound found early use in flavorings, confectionery, and artificial fruit essences—especially banana and pear. In perfumery, it became integral to building fruity top notes and fantasy accords. It was also used in solvents and in the formulation of imitation liqueurs and estery alcoholic notes (Arctander, 1960).
Production Method
Industrial Synthesis:
Isoamyl acetate is synthesized via Fischer esterification, combining isoamyl alcohol (from fractional distillation of fusel oil or petrochemical synthesis) with glacial acetic acid under acidic conditions. The reaction is efficient and cost-effective, yielding high-purity ester upon distillation.
Natural Occurrence:
Found in trace amounts in:
Musa spp. (bananas)
Apples, pears, and apricots
Fermented beverages such as beer and rum
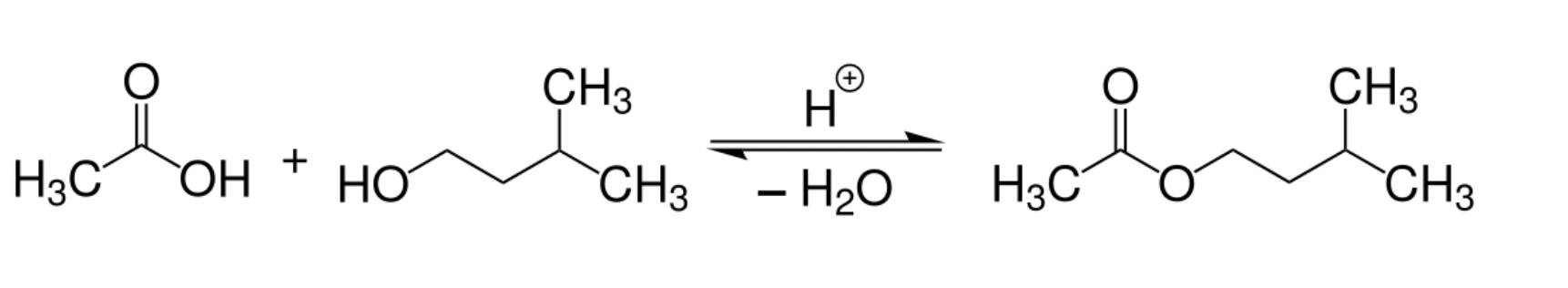
Fischer esterification between isoamyl alcohol and glacial acetic acid.
Olfactory Profile
Odor Description: Intense, fruity, banana-like, with nuances of pear, apple, and solventy sweetness
Intensity: Strong; perceptible at extremely low concentrations
Volatility: Top note
Tenacity: Short to moderate
Threshold: ~0.025 ppm in air (flavor detection)
Arctander (1960) describes it as “the essence of artificial banana flavor,” a foundational component in fruity perfumes and flavorings.
Applications in Perfumery
Isoamyl acetate is essential in:
Fruity top accords (banana, pear, apple)
Tropical or fantasy themes
Playful or retro compositions (e.g., bubblegum or candy-like effects)
Adding estery lift in liqueur-type, cologne, and gourmand bases
Although not typically used in high-end fine fragrance in isolation, it supports complexity in fruit-focused creations or novelty scents.
IFRA Status and Safety Considerations
IFRA Status: Not currently restricted (as of IFRA Amendment 51)
Toxicology:
Generally recognized as safe (GRAS) by FEMA
Low skin sensitization potential
Flammable liquid (flash point: ~35 °C)
May cause mild irritation in concentrated form (Burdock, 2010)
EU Regulation: No mandatory allergen labeling required under EU Regulation 1223/2009.
Environmental & Sustainability Aspects
Isoamyl acetate is readily biodegradable and poses low ecotoxicological risk when used in fragrance applications (ECHA, 2024). Because it can be produced via bio-based fermentation routes (e.g., yeast fermentation of carbohydrates), it offers sustainable sourcing potential for green formulators.
References
Arctander, S. (1960). Perfume and Flavor Chemicals (Aroma Chemicals). Montclair, NJ: Author.
Burdock, G. A. (2010). Fenaroli’s Handbook of Flavor Ingredients (6th ed.). CRC Press.
ECHA. (2024). Isoamyl acetate – Substance Information. European Chemicals Agency. Retrieved from https://echa.europa.eu
Rowan, D. D. (2011). Volatile metabolites. In Annual Plant Reviews: Fruit and Seed Production (Vol. 42, pp. 1–39). Wiley-Blackwell.
Sell, C. S. (2019). The Chemistry of Fragrances (3rd ed.). Royal Society of Chemistry.
Smith, B., March, J., & Carey, F. A. (2006). March’s Advanced Organic Chemistry (6th ed.). Wiley-Interscience.