 Image 1 of 2
Image 1 of 2

 Image 2 of 2
Image 2 of 2



Cyclogalbanate
Synthetic Ingredient for Perfumery
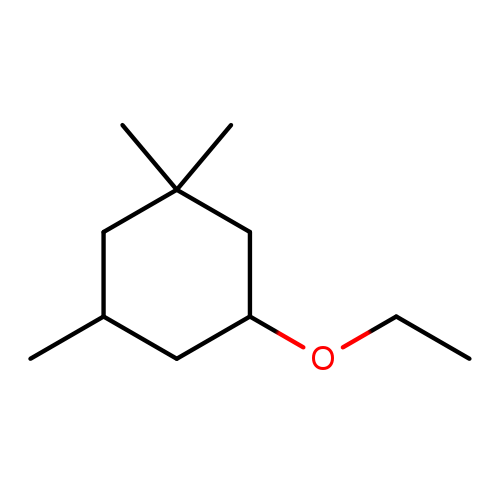
Cyclogalbanate (Allyl (cyclohexyloxy) acetate) is a synthetic green-fruity aroma chemical developed for modern perfumery applications. Known for its distinctive pineapple and galbanum-like profile, this medium-impact ingredient contributes a nuanced green, herbal tone with a mild balsamic and resinous character. Particularly effective in fougère and chypre compositions, Cyclogalbanate is prized for its ability to enhance middle notes, imparting brightness and structure to complex floral-fruity accords.
It is typically used at concentrations between 0.1% and 5%, depending on desired intensity and positioning within the fragrance structure.
Synthetic Ingredient for Perfumery
Cyclogalbanate (Allyl (cyclohexyloxy) acetate) is a synthetic green-fruity aroma chemical developed for modern perfumery applications. Known for its distinctive pineapple and galbanum-like profile, this medium-impact ingredient contributes a nuanced green, herbal tone with a mild balsamic and resinous character. Particularly effective in fougère and chypre compositions, Cyclogalbanate is prized for its ability to enhance middle notes, imparting brightness and structure to complex floral-fruity accords.
It is typically used at concentrations between 0.1% and 5%, depending on desired intensity and positioning within the fragrance structure.
Synthetic Ingredient for Perfumery
Cyclogalbanate (Allyl (cyclohexyloxy) acetate) is a synthetic green-fruity aroma chemical developed for modern perfumery applications. Known for its distinctive pineapple and galbanum-like profile, this medium-impact ingredient contributes a nuanced green, herbal tone with a mild balsamic and resinous character. Particularly effective in fougère and chypre compositions, Cyclogalbanate is prized for its ability to enhance middle notes, imparting brightness and structure to complex floral-fruity accords.
It is typically used at concentrations between 0.1% and 5%, depending on desired intensity and positioning within the fragrance structure.
Synthetic Ingredient Overview – Markdown Format)
🏭 Manufacturer — Symrise
🔎 Chemical name — Allyl (cyclohexyloxy) Acetate
📂 CAS N° — 68901-15-5
⚖️ Molecular Weight — 198.3 g/mol
📝 Odor type — Green → Fruity
📈 Odor Strength — Medium
👃🏼 Odor Profile — Green, pineapple, herbal, slightly resinous and balsamic
⚗️ Uses — Used to enhance floral-fruity accords, particularly in fougère and chypre structures. Elevates middle notes with verdant complexity. Suggested usage: 0.1–5%
🧴 Appearance — Clear to pale yellow liquid with moderate volatility
What is Cyclogalbanate?
Cyclogalbanate, chemically known as Allyl (cyclohexyloxy) acetate, is a synthetic ester introduced in the early 1980s by Hans Warnecke and Ernst Brunke at Dragoco S.A. (now part of Symrise). Their objective was to develop fragrance molecules capable of replicating and extending the olfactory depth of natural materials like galbanum, while offering enhanced stability and versatility.
Cyclogalbanate mimics certain elements of galbanum resin and blends them with pineapple-like fruitiness, creating a hybrid note that is at once green, herbal, and subtly sweet. It plays especially well in fougère, chypre, and floral accords where a softened green accent is desired.
Olfactory Profile and Perfumery Applications
Primary behavior in composition:
Acts as a middle-note enhancer, supporting transitions from citrus or herbal top notes to heavier floral or woody bases
Adds brightness and freshness to otherwise dense structures
Balances aldehydes or synthetic jasmines with a naturalistic green facet
Common applications:
Fougère Fragrances — Harmonizes with lavender, coumarin, and oakmoss
Chypres — Enhances the green-woody interplay of labdanum, patchouli, and citrus
Floral-Fruity Accords — Lifts apple, pineapple, jasmine, and gardenia blends
Functional Products — Suitable for shampoos and detergents with herbal or green profiles due to moderate volatility
Cyclogalbanate is particularly valued in modern perfumery for its ability to replicate the complexity of natural resins like galbanum while maintaining formulation flexibility.
Historical and Industrial Context
Cyclogalbanate was developed during a fertile period of aromatic chemistry at Dragoco S.A. in West Germany (1982–1986). The molecule was part of a wave of green-accent molecules aimed at replacing natural extracts with more sustainable and stable synthetic analogues. Researchers such as Philip Kraft later explored its reactivity and structural compatibility with aldehydes, ionones, and other synthetic florals, confirming its potential as a modular green-building block in fragrance development.
Regulatory and Safety Overview
IFRA Status: Conforms to IFRA standards within recommended usage levels
RIFM Monograph: Available and confirms acceptable toxicological profile at conventional concentrations
GHS Classification: Not classified as hazardous under normal usage
Storage: Store tightly sealed, away from heat sources. Flashpoint > 100°C
Environmental: No major ecotoxicity concerns documented at standard concentrations
Cyclogalbanate is not used in flavors or ingestible products.
Sources
Fulvio Ciccolo, Creative Base Design Notes
Perfumery Raw Materials Handbook, Internal Training (2023)
RIFM Safety Assessment – CAS 68901-15-5
S. Arctander, Perfume and Flavor Chemicals (1960)
Fenaroli’s Handbook of Flavor Ingredients, G.A. Burdock (1995)
FragranceMaterialSafetyResource (Elsevier, 2021)
Formulation Stability Testing Notes – Scentspiracy Studio (2024)